Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19
- PMID: 32692666
- PMCID: PMC7361114
- DOI: 10.1016/j.bios.2020.112437
Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19
Abstract
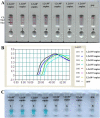
The ongoing global pandemic (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a huge public health issue. Hence, we devised a multiplex reverse transcription loop-mediated isothermal amplification (mRT-LAMP) coupled with a nanoparticle-based lateral flow biosensor (LFB) assay (mRT-LAMP-LFB) for diagnosing COVID-19. Using two LAMP primer sets, the ORF1ab (opening reading frame 1a/b) and N (nucleoprotein) genes of SARS-CoV-2 were simultaneously amplified in a single-tube reaction, and detected with the diagnosis results easily interpreted by LFB. In presence of FITC (fluorescein)-/digoxin- and biotin-labeled primers, mRT-LAMP produced numerous FITC-/digoxin- and biotin-attached duplex amplicons, which were determined by LFB through immunoreactions (FITC/digoxin on the duplex and anti-FITC/digoxin on the test line of LFB) and biotin/treptavidin interaction (biotin on the duplex and strptavidin on the polymerase nanoparticle). The accumulation of nanoparticles leaded a characteristic crimson band, enabling multiplex analysis of ORF1ab and N gene without instrumentation. The limit of detection (LoD) of COVID-19 mRT-LAMP-LFB was 12 copies (for each detection target) per reaction, and no cross-reactivity was generated from non-SARS-CoV-2 templates. The analytical sensitivity of SARS-CoV-2 was 100% (33/33 oropharynx swab samples collected from COVID-19 patients), and the assay's specificity was also 100% (96/96 oropharynx swab samples collected from non-COVID-19 patients). The total diagnostic test can be completed within 1 h from sample collection to result interpretation. In sum, the COVID-19 mRT-LAMP-LFB assay is a promising tool for diagnosing SARS-CoV-2 infections in frontline public health field and clinical laboratories, especially from resource-poor regions.
Keywords: COVID-19; Lateral flow biosensor; Multiplex reverse transcription loop-mediated isothermal amplification; Rapid diagnosis; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Azizi M., Zaferani M., Cheong S.H., Abbaspourrad A. ACS Sens. 2019;4(4):841–848. - PubMed
-
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Euro Surveill. 2020;25(3)
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

