Circadian rhythm influences induction of trained immunity by BCG vaccination
- PMID: 32692732
- PMCID: PMC7641012
- DOI: 10.1172/JCI133934
Circadian rhythm influences induction of trained immunity by BCG vaccination
Abstract
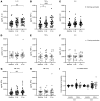
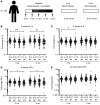
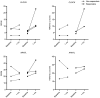
BACKGROUNDThe antituberculosis vaccine bacillus Calmette-Guérin (BCG) reduces overall infant mortality. Induction of innate immune memory, also termed trained immunity, contributes toward protection against heterologous infections. Since immune cells display oscillations in numbers and function throughout the day, we investigated the effect of BCG administration time on the induction of trained immunity.METHODSEighteen volunteers were vaccinated with BCG at 6 pm and compared with 36 age- and sex-matched volunteers vaccinated between 8 am and 9 am. Peripheral blood mononuclear cells were stimulated with Staphylococcus aureus and Mycobacterium tuberculosis before, as well as 2 weeks and 3 months after, BCG vaccination. Cytokine production was measured to assess the induction of trained immunity and adaptive responses, respectively. Additionally, the influence of vaccination time on induction of trained immunity was studied in an independent cohort of 302 individuals vaccinated between 8 am and 12 pm with BCG.RESULTSCompared with evening vaccination, morning vaccination elicited both a stronger trained immunity and adaptive immune phenotype. In a large cohort of 302 volunteers, early morning vaccination resulted in a superior cytokine production capacity compared with later morning. A cellular, rather than soluble, substrate of the circadian effect of BCG vaccination was demonstrated by the enhanced capacity to induce trained immunity in vitro in morning- compared with evening-isolated monocytes.CONCLUSIONSBCG vaccination in the morning induces stronger trained immunity and adaptive responses compared with evening vaccination. Future studies should take vaccine administration time into account when studying specific and nonspecific effects of vaccines; early morning should be the preferred moment of BCG administration.FUNDINGThe Netherlands Organization for Scientific Research, the European Research Council, and the Danish National Research Foundation.
Keywords: Cytokines; Immunology; Innate immunity; Monocytes; Vaccines.
Conflict of interest statement
Figures




Comment in
-
Antituberculosis BCG vaccination: more reasons for varying innate and adaptive immune responses.J Clin Invest. 2020 Oct 1;130(10):5121-5123. doi: 10.1172/JCI141317. J Clin Invest. 2020. PMID: 32813681 Free PMC article.
References
-
- WHO. BCG immunization coverage estimates by WHO region. 2018. Global Health Observatory data repository. https://apps.who.int/gho/data/view.main.81500?lang=en Updated August 26, 2019. Accessed August 4, 2020.
-
- Colditz GA, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. - PubMed
-
- Brewer TF. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31 suppl 3:S64–S67. - PubMed

