Leukemia-Associated Rho Guanine Nucleotide Exchange Factor and Ras Homolog Family Member C Play a Role in Glioblastoma Cell Invasion and Resistance
- PMID: 32693062
- PMCID: PMC7527857
- DOI: 10.1016/j.ajpath.2020.07.005
Leukemia-Associated Rho Guanine Nucleotide Exchange Factor and Ras Homolog Family Member C Play a Role in Glioblastoma Cell Invasion and Resistance
Abstract
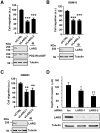
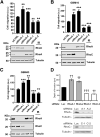
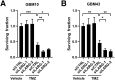
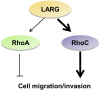
Glioblastoma (GBM) is the most common primary malignant brain cancer in adults. A hallmark of GBM is aggressive invasion of tumor cells into the surrounding normal brain. Both the current standard of care and targeted therapies have largely failed to specifically address this issue. Therefore, identifying key regulators of GBM cell migration and invasion is important. The leukemia-associated Rho guanine nucleotide exchange factor (LARG) has previously been implicated in cell invasion in other tumor types; however, its role in GBM pathobiology remains undefined. Herein, we report that the expression levels of LARG and ras homolog family members C (RhoC), and A (RhoA) increase with glial tumor grade and are highest in GBM. LARG and RhoC protein expression is more prominent in invading cells, whereas RhoA expression is largely restricted to cells in the tumor core. Knockdown of LARG by siRNA inhibits GBM cell migration in vitro and invasion ex vivo in organotypic brain slices. Moreover, siRNA-mediated silencing of RhoC suppresses GBM cell migration in vitro and invasion ex vivo, whereas depletion of RhoA enhances GBM cell migration and invasion, supporting a role for LARG and RhoC in GBM cell migration and invasion. Depletion of LARG increases the sensitivity of GBM cells to temozolomide treatment. Collectively, these results suggest that LARG and RhoC may represent unappreciated targets to inhibit glioma invasion.
Copyright © 2020 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., Di Meco F., Lieberman F., Zhu J.J., Stragliotto G., Tran D., Brem S., Hottinger A., Kirson E.D., Lavy-Shahaf G., Weinberg U., Kim C.Y., Paek S.H., Nicholas G., Bruna J., Hirte H., Weller M., Palti Y., Hegi M.E., Ram Z. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–2316. - PMC - PubMed
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., Hau P., Brandes A.A., Gijtenbeek J., Marosi C., Vecht C.J., Mokhtari K., Wesseling P., Villa S., Eisenhauer E., Gorlia T., Weller M., Lacombe D., Cairncross J.G., Mirimanoff R.O., European Organisation for Research. Treatment of Cancer Brain Tumour. Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Wen P.Y., Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Vega F.M., Ridley A.J. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

