NEAT1 Negatively Regulates Cell Proliferation and Migration of Neuroblastoma Cells by miR-183-5p/FOXP1 Via the ERK/AKT Pathway
- PMID: 32693640
- PMCID: PMC7563027
- DOI: 10.1177/0963689720943608
NEAT1 Negatively Regulates Cell Proliferation and Migration of Neuroblastoma Cells by miR-183-5p/FOXP1 Via the ERK/AKT Pathway
Abstract
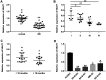
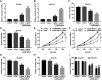
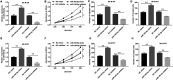
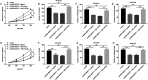
Neuroblastoma, a malignant tumor of the sympathetic nervous system, is an aggressive extracranial tumor in childhood. Long noncoding RNAs (lncRNAs) have been discovered to play a key role in the eukaryotic regulatory gene network and be involved in a wide variety of biological processes. We observed that the expression of lncRNA nuclear-enriched abundant transcript-1 (NEAT1) was significantly decreased in human neuroblastoma tissues and cell lines, compared with the normal. We observed cell proliferation, migration, and invasion with Cell Counting Kit-8 assay, colony formation assay, and Transwell assay to investigate the effects of NEAT1, miR-183-5p, or FOXP1 on neuroblastoma cells. And we also used StarBase and luciferase reporter gene assay to predict and confirm the interaction of NEAT1, miR-183-5p, and FOXP1 in neuroblastoma cells. First, overexpression of NEAT1 suppressed cell proliferation and played a key role in cell migration and invasion. In addition, NEAT1 was demonstrated to directly interact with miR-183-5p and exerted its antioncogenic role in neuroblastoma by negatively regulating miR-183-5p expression. miR-183-5p suppressed the expression of FOXP1 and regulated cell proliferation and migration by directly targeting FOXP1 mRNA 3'-untranslated region. Moreover, FOXP1 antagonized the effect of miR-183-5p on the phosphorylation of extracellular-regulated kinase/protein kinase B (ERK/AKT), while FOXP1 siRNA increased the reduced phosphorylation of ERK/AKT caused by miR-183-5p inhibitor in neuroblastoma cells. Taken together, these data showed that NEAT1 negatively regulated cell proliferation and migration of neuroblastoma by the miR-183-5p/FOXP1 axis via suppression of the ERK/AKT pathway. Our findings may provide a new target for the study of pathogenesis and treatment of neuroblastoma.
Keywords: FOXP1; NEAT1; miR-183-5p; neuroblastoma; the ERK/AKT pathway.
Conflict of interest statement
Figures


Similar articles
-
Long noncoding RNA nuclear enriched abundant transcript 1 impacts cell proliferation, invasion, and migration of glioma through regulating miR-139-5p/ CDK6.J Cell Physiol. 2019 May;234(5):5972-5987. doi: 10.1002/jcp.27093. Epub 2018 Dec 4. J Cell Physiol. 2019. Retraction in: J Cell Physiol. 2023 Feb;238(2):478. doi: 10.1002/jcp.30962. PMID: 30515782 Retracted.
-
LncRNA PVT1 facilitates DLBCL development via miR-34b-5p/Foxp1 pathway.Mol Cell Biochem. 2022 Mar;477(3):951-963. doi: 10.1007/s11010-021-04335-7. Epub 2022 Jan 31. Mol Cell Biochem. 2022. PMID: 35098439
-
Long non-coding RNA NEAT1 promotes the progression of hemangioma via the miR-361-5p/VEGFA pathway.Biochem Biophys Res Commun. 2019 May 14;512(4):825-831. doi: 10.1016/j.bbrc.2019.03.084. Epub 2019 Mar 27. Biochem Biophys Res Commun. 2019. PMID: 30928097
-
Non-Coding RNAs in the Etiology and Control of Major and Neglected Human Tropical Diseases.Front Immunol. 2021 Oct 19;12:703936. doi: 10.3389/fimmu.2021.703936. eCollection 2021. Front Immunol. 2021. PMID: 34737736 Free PMC article. Review.
-
Defining the transcriptional routes controlling lncRNA NEAT1 expression: implications in cellular stress response, inflammation, and differentiation.Discov Oncol. 2025 May 15;16(1):768. doi: 10.1007/s12672-025-02510-6. Discov Oncol. 2025. PMID: 40369379 Free PMC article. Review.
Cited by
-
Integrated single-cell and bulk RNA sequencing analysis establishes a cancer associated fibroblast-related signature for predicting prognosis and therapeutic responses in neuroblastoma.Discov Oncol. 2025 Jul 1;16(1):1235. doi: 10.1007/s12672-025-03023-y. Discov Oncol. 2025. PMID: 40591170 Free PMC article.
-
lncRNA LINC00960 promotes apoptosis by sponging ubiquitin ligase Nrdp1-targeting miR-183-5p.Acta Biochim Biophys Sin (Shanghai). 2023 Jan 25;55(1):91-102. doi: 10.3724/abbs.2023005. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 36722261 Free PMC article.
-
Long Non-Coding RNAs in Neuroblastoma: Pathogenesis, Biomarkers and Therapeutic Targets.Int J Mol Sci. 2024 May 23;25(11):5690. doi: 10.3390/ijms25115690. Int J Mol Sci. 2024. PMID: 38891878 Free PMC article. Review.
-
MicroRNA-183 cluster: a promising biomarker and therapeutic target in gastrointestinal malignancies.Am J Cancer Res. 2023 Dec 15;13(12):6147-6175. eCollection 2023. Am J Cancer Res. 2023. PMID: 38187051 Free PMC article. Review.
-
The role of long non-coding RNA FGD5-AS1 in cancer.Bioengineered. 2022 Apr;13(4):11026-11041. doi: 10.1080/21655979.2022.2067292. Bioengineered. 2022. PMID: 35475392 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

