Tocilizumab for patients with severe COVID-19: a retrospective, multi-center study
- PMID: 32693650
- PMCID: PMC7441800
- DOI: 10.1080/14787210.2020.1800453
Tocilizumab for patients with severe COVID-19: a retrospective, multi-center study
Abstract
Background: Tocilizumab, an inhibitor of the interleukin-6 receptor, may decrease the inflammatory response and control the symptoms of severe coronavirus disease 2019 (COVID-19), but the evidence is scarce.
Methods: This retrospective study included patients with severe COVID-19 requiring oxygen therapy who received tocilizumab in seven centers across Poland. We assessed on-treatment changes in clinical status and inflammatory markers.
Results: Twenty-eight patients were included (19 male), with a mean age of 61.7 ± 12.4 years. The mean time from symptom onset to the first tocilizumab dose was 10.5 ± 5.7 days. Clinical status improved within 24 hours in 11 (39%) patients, within one week in 23 (82%) patients, and within two weeks in 25 (89%); one (4%) patient showed no change and two (7%) patients died. Sixteen patients (57%) no longer needed oxygen therapy within a week (p < 0.001). The serum concentrations of C-reactive protein, procalcitonin, and fibrinogen decreased significantly (p ≤ 0.001). Lung changes improved in 21 (84%) patients within two weeks of treatment; 19 had minimal or no changes upon final examination.
Conclusions: Tocilizumab can control the symptoms of severe COVID-19 by reducing the inflammatory response and rapidly improves the clinical status in most patients.
Keywords: COVID-19; SARS-CoV-2; interleukin-6; therapy; tocilizumab.
Figures
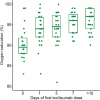


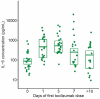

References
-
- The World Health Organization. Coronavirus disease . 2019. (COVID-19) situation report – 159. [cited 2020 June20]. Avialable from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369:m1375. - PubMed
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
