Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma
- PMID: 32694044
- PMCID: PMC7333597
- DOI: 10.1016/j.transci.2020.102871
Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma
Abstract
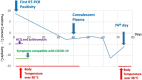
Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first identified in Wuhan, China; and spread all over the world. Reverse-transcription polymerase chain reaction (RT-PCR) test for SARS-CoV-2 usually returns to negative in 20 days post-infection, but prolonged positivity has been reported up to 63 days. A case whose viral shedding lasted 60 days is reported from China. Herein we report a patient with a history of autologous stem cell transplantation (ASCT) for lymphoma whose RT-PCR test remained positive for SARS-CoV-2 for 74 days. The prolonged RT-PCR positivity, despite convalescent plasma infusion, may suggest that the given antibodies may be ineffective in terms of viral clearance. In patients with hematological malignancies or immunosuppression, such as ASCT, may lead to prolonged viral shedding, and strict isolation is warranted for long-term SARS-CoV-2 infection control.
Keywords: Autologous stem cell transplantation (ASCT); COVID-19; Convalescent plasma; Lymphoma; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous