Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites
- PMID: 32694178
- PMCID: PMC7948195
- DOI: 10.1136/gutjnl-2019-319664
Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites
Abstract
Objective: Non-alcoholic fatty liver disease (NAFLD)-associated hepatocellular carcinoma (HCC) is an increasing healthcare burden worldwide. We examined the role of dietary cholesterol in driving NAFLD-HCC through modulating gut microbiota and its metabolites.
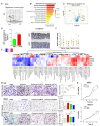
Design: High-fat/high-cholesterol (HFHC), high-fat/low-cholesterol or normal chow diet was fed to C57BL/6 male littermates for 14 months. Cholesterol-lowering drug atorvastatin was administered to HFHC-fed mice. Germ-free mice were transplanted with stools from mice fed different diets to determine the direct role of cholesterol modulated-microbiota in NAFLD-HCC. Gut microbiota was analysed by 16S rRNA sequencing and serum metabolites by liquid chromatography-mass spectrometry (LC-MS) metabolomic analysis. Faecal microbial compositions were examined in 59 hypercholesterolemia patients and 39 healthy controls.
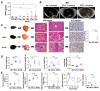
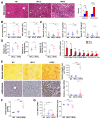
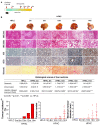
Results: High dietary cholesterol led to the sequential progression of steatosis, steatohepatitis, fibrosis and eventually HCC in mice, concomitant with insulin resistance. Cholesterol-induced NAFLD-HCC formation was associated with gut microbiota dysbiosis. The microbiota composition clustered distinctly along stages of steatosis, steatohepatitis and HCC. Mucispirillum, Desulfovibrio, Anaerotruncus and Desulfovibrionaceae increased sequentially; while Bifidobacterium and Bacteroides were depleted in HFHC-fed mice, which was corroborated in human hypercholesteremia patients. Dietary cholesterol induced gut bacterial metabolites alteration including increased taurocholic acid and decreased 3-indolepropionic acid. Germ-free mice gavaged with stools from mice fed HFHC manifested hepatic lipid accumulation, inflammation and cell proliferation. Moreover, atorvastatin restored cholesterol-induced gut microbiota dysbiosis and completely prevented NAFLD-HCC development.
Conclusions: Dietary cholesterol drives NAFLD-HCC formation by inducing alteration of gut microbiota and metabolites in mice. Cholesterol inhibitory therapy and gut microbiota manipulation may be effective strategies for NAFLD-HCC prevention.
Keywords: dietary factors; fatty liver; intestinal microbiology; nonalcoholic steatohepatitis.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
