The Combined Use of Tocilizumab and Hemoadsorption in a Patient with SARS-COV-2-19-Associated Pneumonia: A Case Report
- PMID: 32694244
- PMCID: PMC7445375
- DOI: 10.1159/000509738
The Combined Use of Tocilizumab and Hemoadsorption in a Patient with SARS-COV-2-19-Associated Pneumonia: A Case Report
Abstract
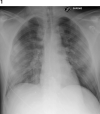
The SARS-COV-2-19-associated respiratory involvement is caused by the massive release of inflammatory cytokines ultimately leading to interstitial pneumonia and acute respiratory distress syndrome (ARDS). In the absence of an effective antiviral treatment, a reasonable causal approach could be constituted by the neutralization of these substances. The authors describe the clinical course of a patient with SARS-COV-2-19 interstitial pneumonia treated with the combination of an anti-interleukin 6 (IL-6) agent (tocilizumab) and hemoadsorption (HA). This combination was used to abate the surge of inflammatory mediators leading to the lung damage. Blood levels of IL-6 and C-reactive protein (CRP) were measured before the initiation of the treatment and in the following 3 days. At the end of the treatment, the values of IL-6 and CRP decreased from 1,040 to 415 pg/mL and from 229 to 59 mg/L, respectively. The gas exchanges and the chest imaging rapidly improved, and the patient was extubated 10 days later. The combination of tocilizumab and HA could be valuable in the treatment of SARS-COV-2-19-associated pneumonia and ARDS that are caused by the release of inflammatory mediators.
Keywords: C-reactive protein.; CytoSorb; Interleukin 6; SARS-COV-2-19; Tocilizumab.
© 2020 S. Karger AG, Basel.
Conflict of interest statement
The authors have non conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous