Metabolic coessentiality mapping identifies C12orf49 as a regulator of SREBP processing and cholesterol metabolism
- PMID: 32694732
- PMCID: PMC7384252
- DOI: 10.1038/s42255-020-0206-9
Metabolic coessentiality mapping identifies C12orf49 as a regulator of SREBP processing and cholesterol metabolism
Abstract
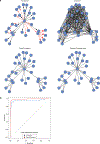
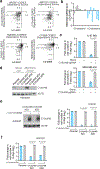
Coessentiality mapping has been useful to systematically cluster genes into biological pathways and identify gene functions1-3. Here, using the debiased sparse partial correlation (DSPC) method3, we construct a functional coessentiality map for cellular metabolic processes across human cancer cell lines. This analysis reveals 35 modules associated with known metabolic pathways and further assigns metabolic functions to unknown genes. In particular, we identify C12orf49 as an essential regulator of cholesterol and fatty acid metabolism in mammalian cells. Mechanistically, C12orf49 localizes to the Golgi, binds membrane-bound transcription factor peptidase, site 1 (MBTPS1, site 1 protease) and is necessary for the cleavage of its substrates, including sterol regulatory element binding protein (SREBP) transcription factors. This function depends on the evolutionarily conserved uncharacterized domain (DUF2054) and promotes cell proliferation under cholesterol depletion. Notably, c12orf49 depletion in zebrafish blocks dietary lipid clearance in vivo, mimicking the phenotype of mbtps1 mutants. Finally, in an electronic health record (EHR)-linked DNA biobank, C12orf49 is associated with hyperlipidaemia through phenome analysis. Altogether, our findings reveal a conserved role for C12orf49 in cholesterol and lipid homeostasis and provide a platform to identify unknown components of other metabolic pathways.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

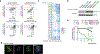
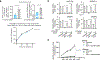







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

