PTH hypersecretion triggered by a GABAB1 and Ca2+-sensing receptor heterocomplex in hyperparathyroidism
- PMID: 32694772
- PMCID: PMC7377265
- DOI: 10.1038/s42255-020-0175-z
PTH hypersecretion triggered by a GABAB1 and Ca2+-sensing receptor heterocomplex in hyperparathyroidism
Abstract
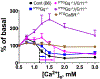
Molecular mechanisms mediating tonic secretion of parathyroid hormone (PTH) in response to hypocalcaemia and hyperparathyroidism (HPT) are unclear. Here we demonstrate increased heterocomplex formation between the calcium-sensing receptor (CaSR) and metabotropic γ-aminobutyric acid (GABA) B1 receptor (GABAB1R) in hyperplastic parathyroid glands (PTGs) of patients with primary and secondary HPT. Targeted ablation of GABAB1R or glutamic acid decarboxylase 1 and 2 in PTGs produces hypocalcaemia and hypoparathyroidism, and prevents PTH hypersecretion in PTGs cultured from mouse models of hereditary HPT and dietary calcium-deficiency. Cobinding of the CaSR/GABAB1R complex by baclofen and high extracellular calcium blocks the coupling of heterotrimeric G-proteins to homomeric CaSRs in cultured cells and promotes PTH secretion in cultured mouse PTGs. These results combined with the ability of PTG to synthesize GABA support a critical autocrine action of GABA/GABAB1R in mediating tonic PTH secretion of PTGs and ascribe aberrant activities of CaSR/GABAB1R heteromer to HPT.
Figures










Similar articles
-
Sex and age modify biochemical and skeletal manifestations of chronic hyperparathyroidism by altering target organ responses to Ca2+ and parathyroid hormone in mice.J Bone Miner Res. 2013 May;28(5):1087-100. doi: 10.1002/jbmr.1846. J Bone Miner Res. 2013. PMID: 23239173 Free PMC article.
-
β-Amyloid as a new target to suppress tonic PTH hypersecretion in primary hyperparathyroidism.medRxiv [Preprint]. 2025 May 28:2025.05.27.25328314. doi: 10.1101/2025.05.27.25328314. medRxiv. 2025. PMID: 40492090 Free PMC article. Preprint.
-
An optogenetic approach for regulating human parathyroid hormone secretion.Nat Commun. 2022 Feb 9;13(1):771. doi: 10.1038/s41467-022-28472-9. Nat Commun. 2022. PMID: 35140213 Free PMC article.
-
Recent updates on the calcium-sensing receptor as a drug target.Curr Med Chem. 2008;15(2):178-86. doi: 10.2174/092986708783330601. Curr Med Chem. 2008. PMID: 18220773 Review.
-
Calcimimetics and hyperparathyroidism.Curr Opin Investig Drugs. 2004 Oct;5(10):1080-5. Curr Opin Investig Drugs. 2004. PMID: 15535429 Review.
Cited by
-
Digital spatial profiling of human parathyroid tumors reveals cellular and molecular alterations linked to vitamin D deficiency.PNAS Nexus. 2023 Mar 9;2(3):pgad073. doi: 10.1093/pnasnexus/pgad073. eCollection 2023 Mar. PNAS Nexus. 2023. PMID: 36992820 Free PMC article.
-
Small vs. Large Library Docking for Positive Allosteric Modulators of the Calcium Sensing Receptor.bioRxiv [Preprint]. 2024 Mar 6:2023.12.27.573448. doi: 10.1101/2023.12.27.573448. bioRxiv. 2024. Update in: Science. 2024 Sep 20;385(6715):eado1868. doi: 10.1126/science.ado1868. PMID: 38234749 Free PMC article. Updated. Preprint.
-
Ex Vivo Intact Tissue Analysis Reveals Alternative Calcium-sensing Behaviors in Parathyroid Adenomas.J Clin Endocrinol Metab. 2021 Oct 21;106(11):3168-3183. doi: 10.1210/clinem/dgab524. J Clin Endocrinol Metab. 2021. PMID: 34272844 Free PMC article.
-
Molecular mechanisms of metabotropic GABAB receptor function.Sci Adv. 2021 May 28;7(22):eabg3362. doi: 10.1126/sciadv.abg3362. Print 2021 May. Sci Adv. 2021. PMID: 34049877 Free PMC article. Review.
-
Epidemiology, Pathophysiology, and Genetics of Primary Hyperparathyroidism.J Bone Miner Res. 2022 Nov;37(11):2315-2329. doi: 10.1002/jbmr.4665. Epub 2022 Oct 17. J Bone Miner Res. 2022. PMID: 36245271 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- F32 DK107177/DK/NIDDK NIH HHS/United States
- T32 GM008424/GM/NIGMS NIH HHS/United States
- P30 AR066262/AR/NIAMS NIH HHS/United States
- R01 AR056256/AR/NIAMS NIH HHS/United States
- R01 AR067291/AR/NIAMS NIH HHS/United States
- R01 DK121656/DK/NIDDK NIH HHS/United States
- I01 BX001960/BX/BLRD VA/United States
- I01 BX003453/BX/BLRD VA/United States
- R01 DK116780/DK/NIDDK NIH HHS/United States
- R01 DK122259/DK/NIDDK NIH HHS/United States
- IK6 BX004835/BX/BLRD VA/United States
- R01 DK087688/DK/NIDDK NIH HHS/United States
- P30 AR075055/AR/NIAMS NIH HHS/United States
- R01 DK111427/DK/NIDDK NIH HHS/United States
- R01 DK102495/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

