Functional identity of hypothalamic melanocortin neurons depends on Tbx3
- PMID: 32694784
- PMCID: PMC8291379
- DOI: 10.1038/s42255-018-0028-1
Functional identity of hypothalamic melanocortin neurons depends on Tbx3
Abstract
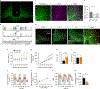
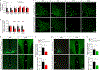
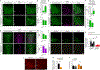
Heterogeneous populations of hypothalamic neurons orchestrate energy balance via the release of specific signatures of neuropeptides. However, how specific intracellular machinery controls peptidergic identities and function of individual hypothalamic neurons remains largely unknown. The transcription factor T-box 3 (Tbx3) is expressed in hypothalamic neurons sensing and governing energy status, whereas human TBX3 haploinsufficiency has been linked with obesity. Here, we demonstrate that loss of Tbx3 function in hypothalamic neurons causes weight gain and other metabolic disturbances by disrupting both the peptidergic identity and plasticity of Pomc/Cart and Agrp/Npy neurons. These alterations are observed after loss of Tbx3 in both immature hypothalamic neurons and terminally differentiated mouse neurons. We further establish the importance of Tbx3 for body weight regulation in Drosophila melanogaster and show that TBX3 is implicated in the differentiation of human embryonic stem cells into hypothalamic Pomc neurons. Our data indicate that Tbx3 directs the terminal specification of neurons as functional components of the melanocortin system and is required for maintaining their peptidergic identity. In summary, we report the discovery of a key mechanistic process underlying the functional heterogeneity of hypothalamic neurons governing body weight and systemic metabolism.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

