An artificial intelligence decision support system for the management of type 1 diabetes
- PMID: 32694787
- PMCID: PMC7384292
- DOI: 10.1038/s42255-020-0212-y
An artificial intelligence decision support system for the management of type 1 diabetes
Abstract
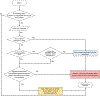
Type 1 diabetes (T1D) is characterized by pancreatic beta cell dysfunction and insulin depletion. Over 40% of people with T1D manage their glucose through multiple injections of long-acting basal and short-acting bolus insulin, so-called multiple daily injections (MDI)1,2. Errors in dosing can lead to life-threatening hypoglycaemia events (<70 mg dl-1) and hyperglycaemia (>180 mg dl-1), increasing the risk of retinopathy, neuropathy, and nephropathy. Machine learning (artificial intelligence) approaches are being harnessed to incorporate decision support into many medical specialties. Here, we report an algorithm that provides weekly insulin dosage recommendations to adults with T1D using MDI therapy. We employ a unique virtual platform3 to generate over 50,000 glucose observations to train a k-nearest neighbours4 decision support system (KNN-DSS) to identify causes of hyperglycaemia or hypoglycaemia and determine necessary insulin adjustments from a set of 12 potential recommendations. The KNN-DSS algorithm achieves an overall agreement with board-certified endocrinologists of 67.9% when validated on real-world human data, and delivers safe recommendations, per endocrinologist review. A comparison of inter-physician-recommended adjustments to insulin pump therapy indicates full agreement of 41.2% among endocrinologists, which is consistent with previous measures of inter-physician agreement (41-45%)5. In silico3,6 benchmarking using a platform accepted by the United States Food and Drug Administration for evaluation of artificial pancreas technologies indicates substantial improvement in glycaemic outcomes after 12 weeks of KNN-DSS use. Our data indicate that the KNN-DSS allows for early identification of dangerous insulin regimens and may be used to improve glycaemic outcomes and prevent life-threatening complications in people with T1D.
Trial registration: ClinicalTrials.gov NCT03443713.
Figures
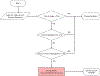

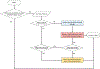
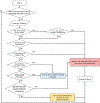
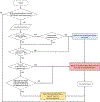




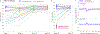
References
-
- Cover T & Hart P Nearest neighbor pattern classification. IEEE Trans. Inf. Theor. 13, 21–27, doi:10.1109/tit.1967.1053964 (2006). - DOI
References (Methods Only)
-
- Whitney AW A Direct Method of Nonparametric Measurement Selection. IEEE Trans. Comput. 20, 1100–1103, doi:10.1109/t-c.1971.223410 (1971). - DOI
-
- Scheiner G Practical CGM: improving patient outcomes through continuous glucose monitoring. (American Diabetes Association, 2015).
-
- White JW, Rassweiler A, Samhouri JF, Stier AC & White C Ecologists should not use statistical significance tests to interpret simulation model results. Oikos 123, 385–388, doi:10.1111/j.1600-0706.2013.01073.x (2014). - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

