Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age
- PMID: 32694825
- PMCID: PMC7438316
- DOI: 10.1038/s42255-020-0228-3
Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age
Abstract
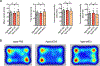
Adipose tissue eosinophils (ATEs) are important in the control of obesity-associated inflammation and metabolic disease. However, the way in which ageing impacts the regulatory role of ATEs remains unknown. Here, we show that ATEs undergo major age-related changes in distribution and function associated with impaired adipose tissue homeostasis and systemic low-grade inflammation in both humans and mice. We find that exposure to a young systemic environment partially restores ATE distribution in aged parabionts and reduces adipose tissue inflammation. Approaches to restore ATE distribution using adoptive transfer of eosinophils from young mice into aged recipients proved sufficient to dampen age-related local and systemic low-grade inflammation. Importantly, restoration of a youthful systemic milieu by means of eosinophil transfers resulted in systemic rejuvenation of the aged host, manifesting in improved physical and immune fitness that was partially mediated by eosinophil-derived IL-4. Together, these findings support a critical function of adipose tissue as a source of pro-ageing factors and uncover a new role of eosinophils in promoting healthy ageing by sustaining adipose tissue homeostasis.
Conflict of interest statement
Figures





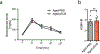






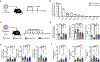
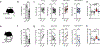




References
-
- Finkel T The metabolic regulation of aging. Nat Med 21, 1416–1423 (2015). - PubMed
-
- López-Otín C, Galluzzi L, Freije JMP, Madeo F & Kroemer G Metabolic Control of Longevity. Cell 166, 802–821 (2016). - PubMed
-
- Gabriely I et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 51, 2951–2958 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

