Chronic Intermittent Ethanol Exposure Increases Ethanol Consumption Following Traumatic Stress Exposure in Mice
- PMID: 32694985
- PMCID: PMC7338656
- DOI: 10.3389/fnbeh.2020.00114
Chronic Intermittent Ethanol Exposure Increases Ethanol Consumption Following Traumatic Stress Exposure in Mice
Abstract
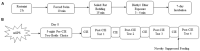
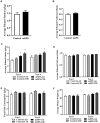
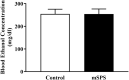
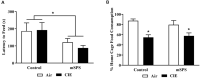
Individuals with post-traumatic stress disorder (PTSD) often use alcohol to cope with their distress. This aberrant use of alcohol often develops into alcohol use disorder (AUD) leading to high rates of PTSD-AUD co-occurrence. Individuals with comorbid PTSD-AUD have more intense alcohol cravings and increased relapse rates during withdrawal than those with AUD alone. Also, individuals with PTSD or AUD alone often show similar psychological behaviors, such as impulsivity and anhedonia. Extensive clinical studies on the behavioral effects of PTSD-AUD comorbidity, namely alcohol use, have been performed. However, these effects have not been well studied or mechanistically explored in animal models. Therefore, the present study evaluated the effects of traumatic stress comorbid with alcohol exposures on ethanol intake, impulsivity, and anhedonia in mice. Adult male C57Bl/6 mice were first exposed to either mouse single-prolonged stress (mSPS), an animal model that has been validated for characteristics akin to PTSD symptoms, or control conditions. Baseline two-bottle choice ethanol consumption and preference tests were conducted after a 7-day isolation period, as part of the mSPS exposure. Next, mice were exposed to air or chronic intermittent ethanol (CIE), a vapor-induced ethanol dependence and withdrawal model, for 4 weeks. Two-bottle choice ethanol drinking was used to measure dependence-induced ethanol consumption and preference during periods intervening CIE cycles. The novelty suppressed feeding (NSF) test was used to evaluate impulsivity and anhedonia behaviors 48 h after mSPS and/or repeated CIE exposure. Results showed that, compared to control conditions, mSPS did not affect baseline ethanol consumption and preference. However, mSPS-CIE mice increased Post-CIE ethanol consumption compared to Control-Air mice. Mice exposed to mSPS had a shorter latency to feed during the NSF, whereas CIE-exposed mice consumed less palatable food reward in their home cage after the NSF. These results demonstrate that mice exposed to both mSPS and CIE are more vulnerable to ethanol withdrawal effects, and those exposed to mSPS have increased impulsivity, while CIE exposure increases anhedonia. Future studies to examine the relationship between behavioral outcomes and the molecular mechanisms in the brain after PTSD-AUD are warranted.
Keywords: alcohol use disorder; anhedonia; chronic intermittent ethanol; ethanol consumption; impulsivity; mouse single-prolonged stress; post-traumatic stress disorder.
Copyright © 2020 Piggott, Lloyd, Perrine and Conti.
Figures
Similar articles
-
Chronic Intermittent Ethanol Vapor Exposure Paired with Two-Bottle Choice to Model Alcohol Use Disorder.J Vis Exp. 2023 Jun 23;(196):10.3791/65320. doi: 10.3791/65320. J Vis Exp. 2023. PMID: 37427930 Free PMC article.
-
Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain.Molecules. 2021 Apr 6;26(7):2086. doi: 10.3390/molecules26072086. Molecules. 2021. PMID: 33917316 Free PMC article.
-
Intermittent Access to Ethanol Drinking Facilitates the Transition to Excessive Drinking After Chronic Intermittent Ethanol Vapor Exposure.Alcohol Clin Exp Res. 2017 Aug;41(8):1502-1509. doi: 10.1111/acer.13434. Epub 2017 Jul 5. Alcohol Clin Exp Res. 2017. PMID: 28679148 Free PMC article.
-
Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder.Genes Brain Behav. 2017 Jan;16(1):15-43. doi: 10.1111/gbb.12349. Epub 2016 Nov 18. Genes Brain Behav. 2017. PMID: 27749004 Free PMC article. Review.
-
Pharmacotherapy for Co-Occurring Alcohol Use Disorder and Post-Traumatic Stress Disorder: Targeting the Opioidergic, Noradrenergic, Serotonergic, and GABAergic/Glutamatergic Systems.Alcohol Res. 2018;39(2):193-205. Alcohol Res. 2018. PMID: 31198658 Free PMC article. Review.
Cited by
-
Long-Term Overconsumption of Sugar Starting at Adolescence Produces Persistent Hyperactivity and Neurocognitive Deficits in Adulthood.Front Neurosci. 2021 Jun 7;15:670430. doi: 10.3389/fnins.2021.670430. eCollection 2021. Front Neurosci. 2021. PMID: 34163325 Free PMC article.
-
Sex differences in basal motivated behavior, chronic ethanol drinking, and amygdala activity in female and male mice.Alcohol. 2024 Nov;120:85-97. doi: 10.1016/j.alcohol.2024.06.004. Epub 2024 Jun 13. Alcohol. 2024. PMID: 38878875
-
Chronic Intermittent Ethanol Vapor Exposure Paired with Two-Bottle Choice to Model Alcohol Use Disorder.J Vis Exp. 2023 Jun 23;(196):10.3791/65320. doi: 10.3791/65320. J Vis Exp. 2023. PMID: 37427930 Free PMC article.
-
Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain.Molecules. 2021 Apr 6;26(7):2086. doi: 10.3390/molecules26072086. Molecules. 2021. PMID: 33917316 Free PMC article.
-
Chronic ethanol induces a pro-inflammatory switch in interleukin-1β regulation of GABAergic signaling in the medial prefrontal cortex of male mice.Brain Behav Immun. 2023 May;110:125-139. doi: 10.1016/j.bbi.2023.02.020. Epub 2023 Feb 28. Brain Behav Immun. 2023. PMID: 36863493 Free PMC article.
References
-
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association.
Grants and funding
LinkOut - more resources
Full Text Sources

