Neoantigen-Specific Adoptive Cell Therapies for Cancer: Making T-Cell Products More Personal
- PMID: 32695101
- PMCID: PMC7333784
- DOI: 10.3389/fimmu.2020.01215
Neoantigen-Specific Adoptive Cell Therapies for Cancer: Making T-Cell Products More Personal
Abstract
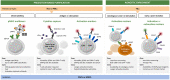
Mutation-derived neoantigens are taking central stage as a determinant in eliciting effective antitumor immune responses following adoptive T-cell therapies. These mutations are patient-specific, and their targeting calls for highly personalized pipelines. The promising clinical outcomes of tumor-infiltrating lymphocyte (TIL) therapy have spurred interest in generating T-cell infusion products that have been selectively enriched in neoantigen (or autologous tumor) reactivity. The implementation of an isolation step, prior to T-cell in vitro expansion and reinfusion, may provide a way to improve the overall response rates achieved to date by adoptive T-cell therapies in metastatic cancer patients. Here we provide an overview of the main technologies [i.e., peptide major histocompatibility complex (pMHC) multimers, cytokine capture, and activation markers] to enrich infiltrating or circulating T-cells in predefined neoantigen specificities (or tumor reactivity). The unique technical and regulatory challenges faced by such highly specialized and patient-specific manufacturing T-cell platforms are also discussed.
Keywords: adoptive cell therapy (ACT); cancer immunotherapy; enrichment; neoantigens; tumor-infiltrating lymphocyte (TIL).
Copyright © 2020 Bianchi, Harari and Coukos.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

