Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation
- PMID: 32695104
- PMCID: PMC7333791
- DOI: 10.3389/fimmu.2020.01261
Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation
Abstract
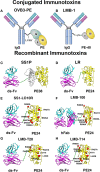
Immunotoxins are cytolytic fusion proteins developed for cancer therapy, composed of an antibody fragment that binds to a cancer cell and a protein toxin fragment that kills the cell. Pseudomonas exotoxin A (PE) is a potent toxin that is used for the killing moiety in many immunotoxins. Moxetumomab Pasudotox (Lumoxiti) contains an anti-CD22 Fv and a 38 kDa portion of PE. Lumoxiti was discovered in the Laboratory of Molecular Biology at the U.S. National Cancer Institute and co-developed with Medimmune/AstraZeneca to treat hairy cell leukemia. In 2018 Lumoxiti was approved by the US Food and Drug Administration for the treatment of drug-resistant Hairy Cell Leukemia. Due to the bacterial origin of the killing moiety, immunotoxins containing PE are highly immunogenic in patients with normal immune systems, but less immunogenic in patients with hematologic malignancies, whose immune systems are often compromised. LMB-100 is a de-immunized variant of the toxin with a humanized antibody that targets mesothelin and a PE toxin that was rationally designed for diminished reactivity with antibodies and B cell receptors. It is now being evaluated in clinical trials for the treatment of mesothelioma and pancreatic cancer and is showing somewhat diminished immunogenicity compared to its un modified parental counterpart. Here we review the immunogenicity of the original and de-immunized PE immunotoxins in mice and patients, the development of anti-drug antibodies (ADAs), their impact on drug availability and their effect on clinical efficacy. Efforts to mitigate the immunogenicity of immunotoxins and its impact on immunogenicity will be described including rational design to identify, remove, or suppress B cell or T cell epitopes, and combination of immunotoxins with immune modulating drugs.
Keywords: B cell epitopes; LMB-100; T cell epitopes; anti-drug antibodies (ADA); moxetumomab pasudotox; neutralizing antibodies; recombinant immunotoxins.
Copyright © 2020 Mazor and Pastan.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

