The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling
- PMID: 32695131
- PMCID: PMC7338657
- DOI: 10.3389/fpls.2020.00968
The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling
Abstract
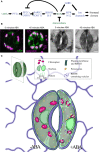
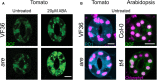
The hormonal and environmental regulation of stomatal aperture is mediated by a complex signaling pathway found within the guard cells that surround stomata. Abscisic acid (ABA) induces stomatal closure in response to drought stress by binding to its guard cell localized receptor, initiating a signaling cascade that includes synthesis of reactive oxygen species (ROS). Genetic evidence in Arabidopsis indicates that ROS produced by plasma membrane respiratory burst oxidase homolog (RBOH) enzymes RBOHD and RBOHF modulate guard cell signaling and stomatal closure. However, ABA-induced ROS accumulates in many locations such as the cytoplasm, chloroplasts, nucleus, and endomembranes, some of which do not coincide with plasma membrane localized RBOHs. ABA-induced guard cell ROS accumulation has distinct spatial and temporal patterns that drive stomatal closure. Productive ROS signaling requires both rapid increases in ROS, as well as the ability of cells to prevent ROS from reaching damaging levels through synthesis of antioxidants, including flavonols. The relationship between locations of ROS accumulation and ABA signaling and the role of enzymatic and small molecule ROS scavengers in maintaining ROS homeostasis in guard cells are summarized in this review. Understanding the mechanisms of ROS production and homeostasis and the role of ROS in guard cell signaling can provide a better understanding of plant response to stress and could provide an avenue for the development of crop plants with increased stress tolerance.
Keywords: abscisic acid; flavonols; guard cell; reactive oxygen species; respiratory burst oxidase homolog; stomata.
Copyright © 2020 Postiglione and Muday.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

