Development and Application of CRISPR/Cas in Microbial Biotechnology
- PMID: 32695770
- PMCID: PMC7338305
- DOI: 10.3389/fbioe.2020.00711
Development and Application of CRISPR/Cas in Microbial Biotechnology
Abstract
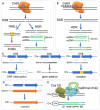
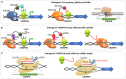
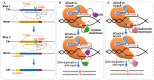
The clustered regularly interspaced short palindromic repeats (CRISPR)-associated (Cas) system has been rapidly developed as versatile genomic engineering tools with high efficiency, accuracy and flexibility, and has revolutionized traditional methods for applications in microbial biotechnology. Here, key points of building reliable CRISPR/Cas system for genome engineering are discussed, including the Cas protein, the guide RNA and the donor DNA. Following an overview of various CRISPR/Cas tools for genome engineering, including gene activation, gene interference, orthogonal CRISPR systems and precise single base editing, we highlighted the application of CRISPR/Cas toolbox for multiplexed engineering and high throughput screening. We then summarize recent applications of CRISPR/Cas systems in metabolic engineering toward production of chemicals and natural compounds, and end with perspectives of future advancements.
Keywords: CRISPR/Cas; gene regulation; genome editing; guide RNA; microbial biotechnology.
Copyright © 2020 Ding, Zhang and Shi.
Figures


Similar articles
-
CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research.Front Med (Lausanne). 2021 Mar 3;8:649896. doi: 10.3389/fmed.2021.649896. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33748164 Free PMC article. Review.
-
CRISPR-cas System as a Genome Engineering Platform: Applications in Biomedicine and Biotechnology.Curr Gene Ther. 2018;18(2):115-124. doi: 10.2174/1566523218666180221110627. Curr Gene Ther. 2018. PMID: 29473500 Review.
-
Application of CRISPR/Cas System in the Metabolic Engineering of Small Molecules.Mol Biotechnol. 2021 Jun;63(6):459-476. doi: 10.1007/s12033-021-00310-1. Epub 2021 Mar 27. Mol Biotechnol. 2021. PMID: 33774733 Review.
-
Applications of CRISPR/Cas System to Bacterial Metabolic Engineering.Int J Mol Sci. 2018 Apr 5;19(4):1089. doi: 10.3390/ijms19041089. Int J Mol Sci. 2018. PMID: 29621180 Free PMC article. Review.
-
CRISPR/Cas System: Recent Advances and Future Prospects for Genome Editing.Trends Plant Sci. 2019 Dec;24(12):1102-1125. doi: 10.1016/j.tplants.2019.09.006. Epub 2019 Nov 11. Trends Plant Sci. 2019. PMID: 31727474 Review.
Cited by
-
Tips, Tricks, and Potential Pitfalls of CRISPR Genome Editing in Saccharomyces cerevisiae.Front Bioeng Biotechnol. 2022 May 30;10:924914. doi: 10.3389/fbioe.2022.924914. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35706506 Free PMC article. Review.
-
CRISPR-Cas Technology: Emerging Applications in Clinical Microbiology and Infectious Diseases.Pharmaceuticals (Basel). 2021 Nov 17;14(11):1171. doi: 10.3390/ph14111171. Pharmaceuticals (Basel). 2021. PMID: 34832953 Free PMC article.
-
Reverse Transcription-Loop-Mediated Isothermal Amplification-CRISPR-Cas13a Technology as a Promising Diagnostic Tool for SARS-CoV-2.Microbiol Spectr. 2022 Oct 26;10(5):e0239822. doi: 10.1128/spectrum.02398-22. Epub 2022 Sep 28. Microbiol Spectr. 2022. PMID: 36169448 Free PMC article.
-
Bacterial live therapeutics for human diseases.Mol Syst Biol. 2024 Dec;20(12):1261-1281. doi: 10.1038/s44320-024-00067-0. Epub 2024 Oct 23. Mol Syst Biol. 2024. PMID: 39443745 Free PMC article. Review.
-
Whole Genome Sequencing Analysis of Effects of CRISPR/Cas9 in Komagataella phaffii: A Budding Yeast in Distress.J Fungi (Basel). 2022 Sep 21;8(10):992. doi: 10.3390/jof8100992. J Fungi (Basel). 2022. PMID: 36294556 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

