The Impact of Di-2-Ethylhexyl Phthalate on Sperm Fertility
- PMID: 32695775
- PMCID: PMC7338605
- DOI: 10.3389/fcell.2020.00426
The Impact of Di-2-Ethylhexyl Phthalate on Sperm Fertility
Abstract
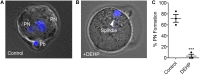
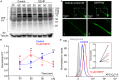
A growing number of studies point to reduced fertility upon chronic exposure to endocrine-disrupting chemicals (EDCs) such as phthalates and plasticizers. These toxins are ubiquitous and are often found in food and beverage containers, medical devices, as well as in common household and personal care items. Animal studies with EDCs, such as phthalates and bisphenol A have shown a dose-dependent decrease in fertility and embryo toxicity upon chronic exposure. However, limited research has been conducted on the acute effects of these EDCs on male fertility. Here we used a murine model to test the acute effects of four ubiquitous environmental toxins: bisphenol A (BPA), di-2-ethylhexyl phthalate (DEHP), diethyl phthalate (DEP), and dimethyl phthalate (DMP) on sperm fertilizing ability and pre-implantation embryo development. The most potent of these toxins, di-2-ethylhexyl phthalate (DEHP), was further evaluated for its effect on sperm ion channel activity, capacitation status, acrosome reaction and generation of reactive oxygen species (ROS). DEHP demonstrated a profound hazardous effect on sperm fertility by producing an altered capacitation profile, impairing the acrosome reaction, and, interestingly, also increasing ROS production. These results indicate that in addition to its known chronic impact on reproductive potential, DEHP also imposes acute and profound damage to spermatozoa, and thus, represents a significant risk to male fertility.
Keywords: acrosome reaction; capacitation; di-2-ethylhexyl phthalate (DEHP); embryo development; endocrine-disrupting chemicals (EDC); phthalates; reactive oxygen species (ROS); spermatozoa.
Copyright © 2020 Khasin, Della Rosa, Petersen, Moeller, Kriegsfeld and Lishko.
Figures



References
-
- Agarwal A., Cocuzza M., Abdelrazik H., Sharma R. K. (2008). Oxidative Stress Measurement in Patients with Male or Female Factor Infertility. Trivandrum: Transworld Research Network.
-
- Aitken R. J., Paterson M., Fisher H., Buckingham D. W., Van Duin M. (1995). Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci. 108 2017–2025. - PubMed

