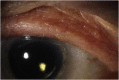
Trichiasis and dry eye syndrome in two patients on novel fibroblast growth factor receptor inhibitor therapies
- PMID: 32695928
- PMCID: PMC7363660
- DOI: 10.1016/j.ajoc.2020.100818
Trichiasis and dry eye syndrome in two patients on novel fibroblast growth factor receptor inhibitor therapies
Abstract
Purpose: Present two patients on two different novel FGFR inhibitors who developed trichiasis with dry eye syndrome.
Observations: Two patients developed trichiasis and dry eye following FGFR inhibitor therapy. Treatments included artificial tears, lifitegrast or cyclosporine, and epilation as needed. One patient discontinued treatment with AZD4547 due to the severity of the ocular adverse effects.
Conclusions and importance: Trichiasis has not yet been reported in patients receiving AZD4547 or INCB054828 treatments and may represent a rare adverse effect of these drugs. Continued research is necessary to determine whether there is a definite link between these FGFR inhibitors and the development of trichiasis.
Keywords: AZD4547; Fibroblast growth factor receptor (FGFR) inhibitor; INCB054828; Pemigatinib; Trichiasis; dry eye.
© 2020 Published by Elsevier Inc.
Conflict of interest statement
The following authors have no financial disclosures: KBV, WMG, CFB, ABL.
Figures
References
-
- Huynh H., Ngo V.C., Fargnoli J. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Canc Res. 2008;14(19):6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. - DOI - PubMed
-
- Andre F., Ranson M., Dean E. Abstract LB-145: results of a phase I study of AZD4547, an inhibitor of fibroblast growth factor receptor (FGFR), in patients with advanced solid tumors. Canc Res. 2013;73(8 suppl ment) doi: 10.1158/1538-7445.AM2013-LB-145. LB-145-LB-145. - DOI
-
- Arkenau H.-T., Saggese M., Hollebecque A. A phase 1 expansion cohort of the fibroblast growth factor receptor (FGFR) inhibitor AZD4547 in patients (pts) with advanced gastric (GC) and gastroesophageal (GOJ) cancer. ASCO Meet Abstr. 2014;32(15_suppl):2620. doi: 10.1200/jco.2014.32.15_suppl.2620. - DOI
-
- Saleh M., Gutierrez M.E., Subbiah V. Abstract CT111: preliminary results from a phase 1/2 study of INCB054828, a highly selective fibroblast growth factor receptor (FGFR) inhibitor, in patients with advanced malignancies. Canc Res. 2017;77(13 suppl ment) doi: 10.1158/1538-7445.AM2017-CT111. CT111-CT111. - DOI
Publication types
LinkOut - more resources
Full Text Sources