Ionizing radiation induces epithelial-mesenchymal transition in human bronchial epithelial cells
- PMID: 32697311
- PMCID: PMC7414515
- DOI: 10.1042/BSR20200453
Ionizing radiation induces epithelial-mesenchymal transition in human bronchial epithelial cells
Abstract
Objective: The present study aimed to analyze the mechanism by which long-term occupational exposure of workers to low-dose ionizing irradiation induces epithelial-mesenchymal transition (EMT) of the human bronchial epithelial cells using transcriptome profiling.
Methods: RNA-seq transcriptomics was used to determine gene expression in blood samples from radiation-exposed workers followed by bioinformatics analysis. Normal bronchial epithelial cells (16HBE) were irradiated for different durations and subjected to immunofluorescence, Western blotting, scratch healing, and adhesion assays to detect the progression of EMT and its underlying molecular mechanisms.
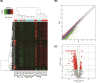
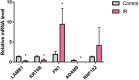
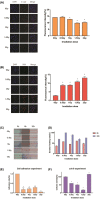
Results: Transcriptomics revealed that exposure to ionizing radiation led to changes in the expression of genes related to EMT, immune response, and migration. At increased cumulative doses, ionizing radiation-induced significant EMT, as evidenced by a gradual decrease in the expression of E-cadherin, increased vimentin, elevated migration ability, and decreased adhesion capability of 16HBE cells. The expression of fibronectin 1 (FN1) showed a gradual increase with the progression of EMT, and may be involved in EMT.
Conclusion: Ionizing radiation induces EMT. FN1 may be involved in the progression of EMT and could serve as a potential biomarker for this process.
Keywords: 16HBE; EMT; FN1; RNA-seq; ionizing radiation.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

