Cumulative deamidations of the major lens protein γS-crystallin increase its aggregation during unfolding and oxidation
- PMID: 32697405
- PMCID: PMC7454558
- DOI: 10.1002/pro.3915
Cumulative deamidations of the major lens protein γS-crystallin increase its aggregation during unfolding and oxidation
Abstract
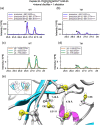
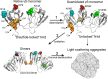
Age-related lens cataract is the major cause of blindness worldwide. The mechanisms whereby crystallins, the predominant lens proteins, assemble into large aggregates that scatter light within the lens, and cause cataract, are poorly understood. Due to the lack of protein turnover in the lens, crystallins are long-lived. A major crystallin, γS, is heavily modified by deamidation, in particular at surface-exposed N14, N76, and N143 to introduce negative charges. In this present study, deamidated γS was mimicked by mutation with aspartate at these sites and the effect on biophysical properties of γS was assessed via dynamic light scattering, chemical and thermal denaturation, hydrogen-deuterium exchange, and susceptibility to disulfide cross-linking. Compared with wild type γS, a small population of each deamidated mutant aggregated rapidly into large, light-scattering species that contributed significantly to the total scattering. Under partially denaturing conditions in guanidine hydrochloride or elevated temperature, deamidation led to more rapid unfolding and aggregation and increased susceptibility to oxidation. The triple mutant was further destabilized, suggesting that the effects of deamidation were cumulative. Molecular dynamics simulations predicted that deamidation augments the conformational dynamics of γS. We suggest that these perturbations disrupt the native disulfide arrangement of γS and promote the formation of disulfide-linked aggregates. The lens-specific chaperone αA-crystallin was poor at preventing the aggregation of the triple mutant. It is concluded that surface deamidations cause minimal structural disruption individually, but cumulatively they progressively destabilize γS-crystallin leading to unfolding and aggregation, as occurs in aged and cataractous lenses.
Keywords: cataracts; crystallins; deamidation; dynamic and static light scattering; hydrogen-deuterium exchange; mass spectrometry; oxidation; protein aggregation; protein unfolding.
© 2020 The Protein Society.
Conflict of interest statement
The authors have no conflict of interests to declare.
Figures
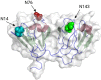



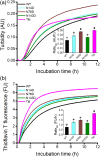
 ,
,  , and
, and  ) denote groups of ratet50 values that are significantly different from each other (p‐value ≤.05)
) denote groups of ratet50 values that are significantly different from each other (p‐value ≤.05)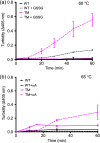

References
-
- Delaye M, Tardieu A. Short‐range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. - PubMed
-
- Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

