Developmental loss of neurofibromin across distributed neuronal circuits drives excessive grooming in Drosophila
- PMID: 32697780
- PMCID: PMC7398555
- DOI: 10.1371/journal.pgen.1008920
Developmental loss of neurofibromin across distributed neuronal circuits drives excessive grooming in Drosophila
Abstract
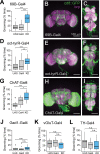
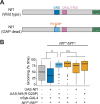
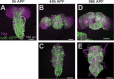
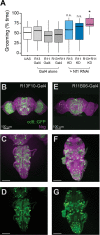
Neurofibromatosis type 1 is a monogenetic disorder that predisposes individuals to tumor formation and cognitive and behavioral symptoms. The neuronal circuitry and developmental events underlying these neurological symptoms are unknown. To better understand how mutations of the underlying gene (NF1) drive behavioral alterations, we have examined grooming in the Drosophila neurofibromatosis 1 model. Mutations of the fly NF1 ortholog drive excessive grooming, and increased grooming was observed in adults when Nf1 was knocked down during development. Furthermore, intact Nf1 Ras GAP-related domain signaling was required to maintain normal grooming. The requirement for Nf1 was distributed across neuronal circuits, which were additive when targeted in parallel, rather than mapping to discrete microcircuits. Overall, these data suggest that broadly-distributed alterations in neuronal function during development, requiring intact Ras signaling, drive key Nf1-mediated behavioral alterations. Thus, global developmental alterations in brain circuits/systems function may contribute to behavioral phenotypes in neurofibromatosis type 1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Eijk S, Mous SE, Dieleman GC, Dierckx B, Rietman AB, de Nijs PFA, Ten Hoopen LW, van Minkelen R, Elgersma Y, Catsman-Berrevoets CE, Oostenbrink R, Legerstee JS. (2018) Autism Spectrum Disorder in an Unselected Cohort of Children with Neurofibromatosis Type 1 (NF1). J Autism Dev Disord, 10.1007/s10803-018-3478-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

