PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation
- PMID: 32697823
- PMCID: PMC7537396
- DOI: 10.1084/jem.20190613
PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation
Abstract
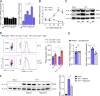
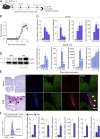
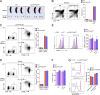
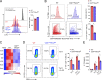
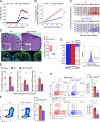
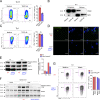
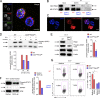
Th17 cell differentiation and pathogenicity depend on metabolic reprogramming inducing shifts toward glycolysis. Here, we show that the pyruvate kinase M2 (PKM2), a glycolytic enzyme required for cancer cell proliferation and tumor progression, is a key factor mediating Th17 cell differentiation and autoimmune inflammation. We found that PKM2 is highly expressed throughout the differentiation of Th17 cells in vitro and during experimental autoimmune encephalomyelitis (EAE) development. Strikingly, PKM2 is not required for the metabolic reprogramming and proliferative capacity of Th17 cells. However, T cell-specific PKM2 deletion impairs Th17 cell differentiation and ameliorates symptoms of EAE by decreasing Th17 cell-mediated inflammation and demyelination. Mechanistically, PKM2 translocates into the nucleus and interacts with STAT3, enhancing its activation and thereby increasing Th17 cell differentiation. Thus, PKM2 acts as a critical nonmetabolic regulator that fine-tunes Th17 cell differentiation and function in autoimmune-mediated inflammation.
© 2020 Damasceno et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






References
-
- Angiari S., Runtsch M.C., Sutton C.E., Palsson-McDermott E.M., Kelly B., Rana N., Kane H., Papadopoulou G., Pearce E.L., Mills K.H.G., et al. . 2020. Pharmacological Activation of Pyruvate Kinase M2 Inhibits CD4+ T Cell Pathogenicity and Suppresses Autoimmunity. Cell Metab. 31:391–405.e8. 10.1016/j.cmet.2019.10.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

