Mechanism of Vascular Toxicity in Rats Subjected to Treatment with a Tyrosine Kinase Inhibitor
- PMID: 32698382
- PMCID: PMC7560282
- DOI: 10.3390/toxics8030049
Mechanism of Vascular Toxicity in Rats Subjected to Treatment with a Tyrosine Kinase Inhibitor
Abstract
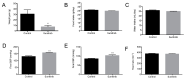
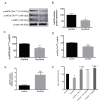
Sunitinib (Su) is a tyrosine kinase inhibitor with antiangiogenic and antineoplastic effects that is recommended therapy for renal cell carcinoma, gastrointestinal stromal tumors, and pancreatic neuroendocrine tumors. Arterial hypertension is one of the adverse effects observed in the treatment with Su. The aim of this work was to deepen our understanding of the underlying mechanisms involved in the development of this side effect. Studies on endothelial function, vascular remodeling and nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) system were carried out in thoracic aortas from rats treated with Su for three weeks. Animals subjected to Su treatment presented with increased blood pressure and reduced endothelium-dependent vasodilation, the latter being reverted by NADPH oxidase blockade. Furthermore, vascular remodeling and stronger Masson trichrome staining, together with enhanced immunofluorescence signal for collagen 1 alpha 1 (Col1α1), were observed in aortas from treated animals. These results were accompanied by a significant elevation in superoxide anion production and the activity/protein/gene expression of NADPH oxidase isoforms (NOX1, NOX2, and NOX4), which was also prevented by NOX inhibition. Furthermore, a decrease in nitric oxide (NO) levels and endothelial nitric oxide synthase (eNOS) activation was observed in aortas from Su-treated animals. All these results indicate that endothelial dysfunction secondary to changes in vascular remodeling and oxidative stress might be responsible for the typical arterial hypertension that develops following treatment with Su.
Keywords: arterial hypertension; endothelial dysfunction; tyrosine kinase inhibitor; vascular remodeling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Faivre S., Niccoli P., Castellano D., Valle J.W., Hammel P., Raoul J.L., Vinik A., Van Cutsem E., Bang Y.J., Lee S.H., et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017 doi: 10.1093/annonc/mdw561. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

