Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver
- PMID: 32698427
- PMCID: PMC7404993
- DOI: 10.3390/toxins12070463
Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver
Abstract
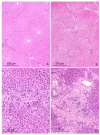
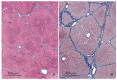
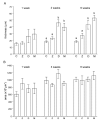
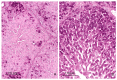
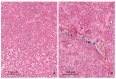
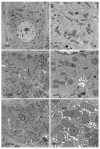
The purpose of this study was to determine the effects of single and combined administrations of deoxynivalenol (DON) and zearalenone (ZEN) on the histology and ultrastructure of pig liver. The study was performed on immature gilts, which were divided into four equal groups. Animals in the experimental groups received DON at a dose of 12 μg/kg body weight (BW) per day, ZEN at 40 μg/kg BW per day, or a mixture of DON (12 μg/kg BW per day) and ZEN (40 μg/kg BW). The control group received vehicle. The animals were killed after 1, 3, and 6 weeks of experiment. Treatment with mycotoxins resulted in several changes in liver histology and ultrastructure, including: (1) an increase in the thickness of the perilobular connective tissue and its penetration to the lobules in gilts receiving DON and DON + ZEN; (2) an increase in the total microscopic liver score (histology activity index (HAI)) in pigs receiving DON and DON + ZEN; (3) dilatation of hepatic sinusoids in pigs receiving ZEN, DON and DON + ZEN; (4) temporary changes in glycogen content in all experimental groups; (5) an increase in iron accumulation in the hepatocytes of gilts treated with ZEN and DON + ZEN; (6) changes in endoplasmic reticulum organization in the hepatocytes of pigs receiving toxins; (7) changes in morphology of Browicz-Kupffer cells after treatment with ZEN, DON, and DON + ZEN. The results show that low doses of mycotoxins used in the present study, even when applied for a short period, affected liver morphology.
Keywords: deoxynivalenol; hepatocyte; histology; liver; mycotoxins; pig; ultrastructure; zearalenone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Nayakwadi S., Ramu R., Kumar Sharma A., Kumar Gupta V., Rajukumar K., Kumar V., Shirahatti P.S., Rashmi L., Basalingappa K.M. Toxicopathological studies on the effects of T-2 mycotoxin and their interaction in juvenile goats. PLoS ONE. 2020;15:e229463. doi: 10.1371/journal.pone.0229463. - DOI - PMC - PubMed
-
- Koselski M., Dziubińska H., Trębacz K., Sieprawska A., Filek M. The Role of SV Ion Channels under the Stress of Mycotoxins Induced in Wheat Cells—Protective Action of Selenium Ions. J. Plant Growth Regul. 2019;38:1255–1259. doi: 10.1007/s00344-019-09930-z. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

