Lactate-Based Model Predictive Control Strategy of Cell Growth for Cell Therapy Applications
- PMID: 32698462
- PMCID: PMC7552707
- DOI: 10.3390/bioengineering7030078
Lactate-Based Model Predictive Control Strategy of Cell Growth for Cell Therapy Applications
Abstract
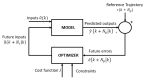
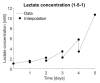
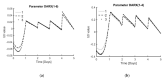
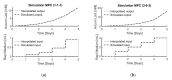
Implementing a personalised feeding strategy for each individual batch of a bioprocess could significantly reduce the unnecessary costs of overfeeding the cells. This paper uses lactate measurements during the cell culture process as an indication of cell growth to adapt the feeding strategy accordingly. For this purpose, a model predictive control is used to follow this a priori determined reference trajectory of cumulative lactate. Human progenitor cells from three different donors, which were cultivated in 12-well plates for five days using six different feeding strategies, are used as references. Each experimental set-up is performed in triplicate and for each run an individualised model-based predictive control (MPC) controller is developed. All process models exhibit an accuracy of 99.80% ± 0.02%, and all simulations to reproduce each experimental run, using the data as a reference trajectory, reached their target with a 98.64% ± 0.10% accuracy on average. This work represents a promising framework to control the cell growth through adapting the feeding strategy based on lactate measurements.
Keywords: advanced therapy medicinal products; bio-process; cell growth; lactate; model predictive control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Machine learning-based model predictive controller design for cell culture processes.Biotechnol Bioeng. 2023 Aug;120(8):2144-2159. doi: 10.1002/bit.28486. Epub 2023 Jul 3. Biotechnol Bioeng. 2023. PMID: 37395526
-
Towards advanced bioprocess optimization: A multiscale modelling approach.Comput Struct Biotechnol J. 2023 Jul 8;21:3639-3655. doi: 10.1016/j.csbj.2023.07.003. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37520284 Free PMC article.
-
Experimental Evaluation on Depth Control Using Improved Model Predictive Control for Autonomous Underwater Vehicle (AUVs).Sensors (Basel). 2018 Jul 17;18(7):2321. doi: 10.3390/s18072321. Sensors (Basel). 2018. PMID: 30018268 Free PMC article.
-
Fed-batch cultivation of animal cells using different medium design concepts and feeding strategies.Biotechnol Bioeng. 1994 May;43(11):1175-89. doi: 10.1002/bit.260431123. Biotechnol Bioeng. 1994. PMID: 18615531
-
Predictive control strategy of a gas turbine for improvement of combined cycle power plant dynamic performance and efficiency.Springerplus. 2016 Jul 4;5(1):980. doi: 10.1186/s40064-016-2679-2. eCollection 2016. Springerplus. 2016. PMID: 28443216 Free PMC article.
Cited by
-
An Overview of Software Sensor Applications in Biosystem Monitoring and Control.Sensors (Basel). 2024 Oct 20;24(20):6738. doi: 10.3390/s24206738. Sensors (Basel). 2024. PMID: 39460218 Free PMC article. Review.
-
Single-Use, Metabolite Absorbing, Resonant Transducer (SMART) Culture Vessels for Label-Free, Continuous Cell Culture Progression Monitoring.Adv Sci (Weinh). 2024 Aug;11(32):e2401260. doi: 10.1002/advs.202401260. Epub 2024 Jun 20. Adv Sci (Weinh). 2024. PMID: 38900081 Free PMC article.
-
A high-density microfluidic bioreactor for the automated manufacturing of CAR T cells.Nat Biomed Eng. 2024 Dec;8(12):1571-1591. doi: 10.1038/s41551-024-01219-1. Epub 2024 Jun 4. Nat Biomed Eng. 2024. PMID: 38834752
-
Stem Cell Bioprocessing and Manufacturing.Bioengineering (Basel). 2020 Jul 31;7(3):84. doi: 10.3390/bioengineering7030084. Bioengineering (Basel). 2020. PMID: 32751782 Free PMC article.
References
-
- European Medicines Agency Advanced Therapy Medicinal Products. [(accessed on 18 May 2020)]; Available online: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-....
-
- Office of Tissues and Advanced Therapies Approved Cellular and Gene Therapy Products|FDA. [(accessed on 17 July 2020)];2020 Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ....
Grants and funding
LinkOut - more resources
Full Text Sources

