Depletion of Macrophages Improves Therapeutic Response to Gemcitabine in Murine Pancreas Cancer
- PMID: 32698524
- PMCID: PMC7409345
- DOI: 10.3390/cancers12071978
Depletion of Macrophages Improves Therapeutic Response to Gemcitabine in Murine Pancreas Cancer
Abstract
Background: The tumor microenvironment (TME) is composed of fibro-inflammatory cells and extracellular matrix (ECM) components. However, the exact contribution of the various TME compartments towards therapeutic response is unknown. Here, we aim to dissect the specific contribution of tumor-associated macrophages (TAMs) towards drug delivery and response in pancreatic ductal adenocarcinoma (PDAC).
Methods: The effect of gemcitabine was assessed in human and murine macrophages, human pancreatic stellate cells (hPSCs), and tumor cells (L3.6pl, BxPC3 and KPC) in vitro. The drug metabolism of gemcitabine was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Preclinical studies were conducted using KrasG12D;p48-Cre and KrasG12D;p53172H;Pdx-Cre mice to investigate gemcitabine delivery at different stages of tumor progression and upon pharmacological TAM depletion.
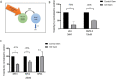
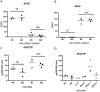
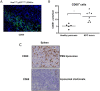
Results: Gemcitabine accumulation was significantly increased in murine PDAC tissue compared to pancreatic intraepithelial neoplasia (PanIN) lesions and healthy control pancreas tissue. In vitro, macrophages accumulated and rapidly metabolized gemcitabine resulting in a significant drug scavenging effect for gemcitabine. Finally, pharmacological TAM depletion enhanced therapeutic response to gemcitabine in tumor-bearing KPC mice.
Conclusion: Macrophages rapidly metabolize gemcitabine in vitro, and pharmacological depletion improves the therapeutic response to gemcitabine in vivo. Our study supports the notion that TAMs might be a promising therapeutic target in PDAC.
Keywords: chemoresistance; drug delivery; macrophages; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Mahajan U.M., Langhoff E., Goni E., Costello E., Greenhalf W., Halloran C., Ormanns S., Kruger S., Boeck S., Ribback S., et al. Immune cell and stromal signature associated with progression-free survival of patients with resected pancreatic ductal adenocarcinoma. Gastroenterology. 2018;155:1625–1639. doi: 10.1053/j.gastro.2018.08.009. - DOI - PubMed
-
- Ozdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S.V., et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. - DOI - PMC - PubMed
-
- Ramu I., Buchholz S.M., Patzak M.S., Goetze R.G., Singh S.K., Richards F.M., Jodrell D.I., Sipos B., Strobel P., Ellenrieder V., et al. SPARC dependent collagen deposition and gemcitabine delivery in a genetically engineered mouse model of pancreas cancer. EBioMedicine. 2019;48:161–168. doi: 10.1016/j.ebiom.2019.09.024. - DOI - PMC - PubMed
-
- Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A., Dekleva E.N., Saunders T., Becerra C.P., Tattersall I.W., et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

