Molecular and Hormonal Regulation of Leaf Morphogenesis in Arabidopsis
- PMID: 32698541
- PMCID: PMC7404056
- DOI: 10.3390/ijms21145132
Molecular and Hormonal Regulation of Leaf Morphogenesis in Arabidopsis
Abstract
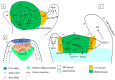
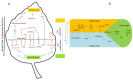
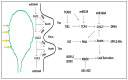
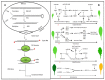
Shoot apical meristems (SAM) are tissues that function as a site of continuous organogenesis, which indicates that a small pool of pluripotent stem cells replenishes into lateral organs. The coordination of intercellular and intracellular networks is essential for maintaining SAM structure and size and also leads to patterning and formation of lateral organs. Leaves initiate from the flanks of SAM and then develop into a flattened structure with variable sizes and forms. This process is mainly regulated by the transcriptional regulators and mechanical properties that modulate leaf development. Leaf initiation along with proper orientation is necessary for photosynthesis and thus vital for plant survival. Leaf development is controlled by different components such as hormones, transcription factors, miRNAs, small peptides, and epigenetic marks. Moreover, the adaxial/abaxial cell fate, lamina growth, and shape of margins are determined by certain regulatory mechanisms. The over-expression and repression of various factors responsible for leaf initiation, development, and shape have been previously studied in several mutants. However, in this review, we collectively discuss how these factors modulate leaf development in the context of leaf initiation, polarity establishment, leaf flattening and shape.
Keywords: adaxial/abaxial; leaf development; miRNAs; shoot apical meristem; transcription factors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cho K.H., Jun S.E., Jeong S.J., Lee Y.K., Kim G.T. Developmental processes of leaf morphogenesis in arabidopsis. J. Plant Biol. 2007;50:282–290. doi: 10.1007/BF03030656. - DOI
-
- Li L.C., Kang D.M., Chen Z.L., Qu L.J. Hormonal regulation of leaf morphogenesis in Arabidopsis. J. Integ. Plant Biol. 2007;49:75–80. doi: 10.1111/j.1744-7909.2006.00410.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

