Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study
- PMID: 32699031
- PMCID: PMC7541683
- DOI: 10.1158/2159-8290.CD-20-0941
Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study
Abstract
Among 2,186 U.S. adults with invasive cancer and laboratory-confirmed SARS-CoV-2 infection, we examined the association of COVID-19 treatments with 30-day all-cause mortality and factors associated with treatment. Logistic regression with multiple adjustments (e.g., comorbidities, cancer status, baseline COVID-19 severity) was performed. Hydroxychloroquine with any other drug was associated with increased mortality versus treatment with any COVID-19 treatment other than hydroxychloroquine or untreated controls; this association was not present with hydroxychloroquine alone. Remdesivir had numerically reduced mortality versus untreated controls that did not reach statistical significance. Baseline COVID-19 severity was strongly associated with receipt of any treatment. Black patients were approximately half as likely to receive remdesivir as white patients. Although observational studies can be limited by potential unmeasured confounding, our findings add to the emerging understanding of patterns of care for patients with cancer and COVID-19 and support evaluation of emerging treatments through inclusive prospective controlled trials. SIGNIFICANCE: Evaluating the potential role of COVID-19 treatments in patients with cancer in a large observational study, there was no statistically significant 30-day all-cause mortality benefit with hydroxychloroquine or high-dose corticosteroids alone or in combination; remdesivir showed potential benefit. Treatment receipt reflects clinical decision-making and suggests disparities in medication access.This article is highlighted in the In This Issue feature, p. 1426.
©2020 American Association for Cancer Research.
Conflict of interest statement
S.L. Peters reports personal fees and other from Roche/Genentech (advisor/consultant role, and satellite symposium, all fees to institution), personal fees and other from BMS (advisor/consultant role, and satellite symposium, all fees to institution), MSD (advisor/consultant role, and satellite symposium, all fees to institution), Merck Serono (advisor/consultant role, all fees to institution), Pfizer (advisor/consultant role, and satellite symposium, all fees to institution), Novartis (advisor/consultant role, and satellite symposium, all fees to institution), AstraZeneca (advisor/consultant role, and satellite symposium, all fees to institution), Regeneron (advisor/consultant role, all fees to institution), Boehringer Ingelheim (advisor/consultant role, and satellite symposium, all fees to institution), Amgen (advisor/consultant role, all fees to institution), Bioinvent (advisor/consultant role, all fees to institution), Daiichi Sankyo (advisor/consultant role, all fees to institution), Biocartis (advisor/consultant role, all fees to institution), AbbVie (advisor/consultant role, all fees to institution), Debiopharm (advisor/consultant role, all fees to institution), Eli Lilly (advisor/consultant role, and satellite symposium, all fees to institution), Foundation Medicine (advisor/consultant role, and satellite symposium, all fees to institution), Illumina (advisor/consultant role, and satellite symposium, all fees to institution), Janssen (advisor/consultant role, all fees to institution), Pharmamar (advisor/consultant role, all fees to institution), Sanofi (advisor/consultant role, and satellite symposium, all fees to institution), Seattle Genetics (advisor/consultant role, all fees to institution), Takeda (advisor/consultant role, and satellite symposium, all fees to institution), Vaccibody (advisor/consultant role, all fees to institution), and Mirati (advisor/consultant role, all fees to institution) outside the submitted work. O.A. Panagiotou reports grants from NCI during the conduct of the study. D.P. Shah reports grants from American Cancer Society and Hope Research Foundation [this work was supported in part by the American Cancer Society and the Hope Foundation for Cancer Research (Mentored Research Scholar Grants in Applied and Clinical Research, MRSG-16-152-01-CCE; to D.P. Shah)] during the conduct of the study. N.M. Kuderer reports personal fees from Celldex (consulting fees), BMS (consulting fees), Janssen (consulting fees), Invitae (consulting fees), Total Health (consulting fees), Beyond Springs (consulting fees), Bayer (consulting fees), and Spectrum Pharmaceuticals (consulting fees) outside the submitted work. B.J. Lee reports grants from National Science Foundation (NSF; his contributions to this manuscript are part of his work as an NSF Research Experience for Undergraduates (REU) student) during the conduct of the study. T.K. Choueiri reports grants, personal fees, nonfinancial support, and other from AstraZeneca (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support), Pfizer (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support), Exelixis (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support), BMS (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support), Merck (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support), Novartis (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support), GSK (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support), and Roche (clinical trials, advisory board, consultancy and related travel/lodging and manuscript support) during the conduct of the study, Pfizer (related to kidney cancer: clinical trials, advisory board, consultancy, manuscript support), Exelixis (related to kidney cancer: clinical trials, advisory board, consultancy, manuscript support), BMS (related to kidney cancer: clinical trials, advisory board, consultancy, manuscript support), Merck (related to kidney cancer: clinical trials, advisory board, consultancy, manuscript support), Roche/Genentech (related to kidney cancer: clinical trials, advisory board, consultancy, manuscript support), and Novartis (related to kidney cancer: clinical trials, advisory board, consultancy, manuscript support) outside the submitted work; and no leadership or employment in for-profit companies. Other present or past leadership roles: Director of GU Oncology Division at Dana-Farber and past President of medical Staff at Dana-Farber), member of NCCN Kidney panel and the GU Steering Committee, past chairman of the Kidney Cancer Association Medical and Scientific Steering Committee, KidneyCan Advisory board, Kidney cancer Research Summit co-chair (2019-present). P. Grivas reports grants and personal fees from Pfizer, Genentech, Bayer, Merck, and Mirati Therapeutics, Bristol-Myers Squibb, and QED Therapeutics; personal fees from EMD Serono, Oncogenex, Seattle Genetics, Foundation Medicine, Driver, Heron Therapeutics, Janssen, GlaxoSmithKline, Genzyme, Roche, and Exelixis; grants, personal fees, and nonfinancial support from AstraZeneca, Clovis Oncology; and grants from Bavarian Nordic, Immunomedics, and Debiopharm, and Kure It Cancer Research outside the submitted work. B.I. Rini reports grants, personal fees, and nonfinancial support from Merck and BMS; grants and personal fees from Pfizer, Arravive, and AVEO; grants from Genentech; personal fees from Surface Oncology, 3D Medicines, Arrowhead outside the submitted work. M.A. Thompson reports personal fees from Adaptive (advisory board, registry), UpToDate (royalties), and AIM Specialty Health (advisory board) outside the submitted work; other from CRAB CTC (institutional), Amgen (institutional), Hoosier Research Network (institutional), Janssen (institutional), Lilly (institutional), LynxBio (institutional), Strata Oncology (institutional), Takeda (institutional), TG Therapeutics (institutional); personal fees and other from BMS (Celgene; advisory board, registry; institutional), Takeda (Celgene; advisory board, registry; institutional), GSK (institutional; advisory board December 12, 2017). Z. Bakouny reports nonfinancial support from Bristol-Myers Squibb and grants from Genentech outside the submitted work. D.B. Doroshow reports grants from NCI [the Tisch Cancer Institute Cancer Center Support Grant (1P30CA196521)] during the conduct of the study; other from Janssen Oncology (institutional funding), Dendreon (institutional funding), Novartis (institutional funding), Bristol-Myers Squibb (institutional funding), Merck (institutional funding), AstraZeneca (institutional funding), and Genentech/Roche (institutional funding) outside the submitted work. P.C. Egan reports research support to her institution from CTI Biopharma Corp. M.D. Galsky reports personal fees from Janssen, GlaxoSmithKline, Lilly, Astellas, Pfizer, EMD Serono, Seattle Genetics, Incyte, Aileron, Dracen, Inovio, NuMab, and Dragonfly outside the submitted work; grants and personal fees from Genentech, Dendreon, Merck, AstraZeneca, Bristol-Myers Squibb; and grants from Novartis. T.F. Halfdanarson reports personal fees from Curium (consulting/advisory board), TERUMO (consulting/advisory board), ScioScientific (consulting/advisory board); nonfinancial support from Ipsen (consulting; fees paid to institution), Advanced Accelerator Applications (consulting; fees paid to institution); grants from Thermo Fisher Scientific (research funding to institution), Basilea (research funding to institution), and Agios (research funding to institution) outside the submitted work. B. Halmos reports grants and personal fees from Merck, BMS, Novartis, Pfizer, Eli Lilly, Boehringer-Ingelheim, AstraZeneca, Guardant Health, Takeda, and Amgen outside the submitted work; and personal fees from Genentech and TPT; grants from AbbVie, Advaxis, and GSK. A.R. Khaki reports grants from NIH (T32CA009515) outside the submitted work. S. Mishra reports grants from NIH (P30 CA068485) during the conduct of the study. A.J. Olszewski reports other from Genentech (research funds for the institution), TG Therapeutics (research funds for the institution); other from Spectrum Pharmaceuticals (research funds for the institution); and nonfinancial support from Adaptive Biotechnologies (research support) outside the submitted work. N.A. Pennell reports personal fees from Merck (advisory board), AstraZeneca (advisory board), Genentech (advisory board), Amgen (advisory board), BMS (advisory board), Eli Lilly (advisory board), G1 Therapeutics (advisory board), and Regeneron (advisory board) outside the submitted work. A. Schmidt reports nonfinancial support from Pfizer and Astellas outside the submitted work. G.K. Schwartz reports personal fees from Apexigen (advisory board), Array (advisory board), Epizyme (advisory board), GenCirq (advisory board), Daiichi Sankyo (advisory board), Fortress (consultant), Iovance Biotherapeutics (consultant), Bayer Pharmaceuticals (sarcoma advisory board), Pfizer Oncology (consultant), Puretech (consultant), PTC Therapeutics (consultant), Ellipsis Pharma (scientific advisory group), and Conarlo (SAB member) outside the submitted work; other from Bionaut (advisory board); personal fees from Oncgoenuity (SAB member); and grants from Astex. Y. Shyr reports grants from NCI during the conduct of the study. G.H. Lyman reports grants and nonfinancial support from Amgen; personal fees from G1 Therapeutics, Invitae, Sandoz, Samsung Bioepi, Beyond Spring, Spectrum, Merck, Mylan, and Partner Therapeutics. J.L. Warner reports grants from NCI (P30 CA068485; U01 CA231840) during the conduct of the study; personal fees from Westat, other from HemOnc.org (stock ownership; no monetary value); and personal fees from IBM Watson Health outside the submitted work. No potential conflicts of interest were disclosed by the other authors.
Figures
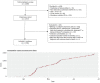


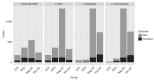
References
-
- RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with covid-19—preliminary report. N Engl J Med 2020. Jul 17 [Epub ahead of print].
-
- Collins R, Bowman L, Landray M, Peto R. The magic of randomization versus the myth of real-world evidence. N Engl J Med 2020;382:674–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

