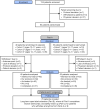
Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial
- PMID: 32699034
- PMCID: PMC7509523
- DOI: 10.1136/annrheumdis-2020-217101
Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial
Abstract
Objectives: This ongoing Phase-2, randomised, placebo-controlled, double-blind study evaluated the efficacy, safety and pharmacokinetics of intravenous belimumab in childhood-onset systemic lupus erythematosus (cSLE).
Methods: Patients (5 to 17 years) were randomised to belimumab 10 mg/kg intravenous or placebo every 4 weeks, plus standard SLE therapy. Primary endpoint: SLE Responder Index (SRI4) response rate (Week 52). Key major secondary endpoints: proportion of patients achieving the Paediatric Rheumatology International Trials Organisation/American College of Rheumatology (PRINTO/ACR) response using 50 and '30 alternative' definitions (Week 52), and sustained response (Weeks 44 to 52) by SRI4 and Parent Global Assessment of well-being (Parent-global). Safety and pharmacokinetics were assessed. Study not powered for statistical testing.
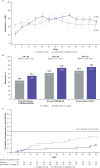
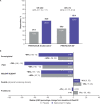
Results: Ninety-three patients were randomised (belimumab, n=53; placebo, n=40). At Week 52, there were numerically more SRI4 responders with belimumab versus placebo (52.8% vs 43.6%; OR 1.49 (95% CI 0.64 to 3.46)). PRINTO/ACR 30 alternative (52.8% vs 27.5%; OR 2.92 (95% CI 1.19 to 7.17)) and PRINTO/ACR 50 (60.4% vs 35.0%; OR 2.74 (95% CI 1.15 to 6.54)) responses were more frequent with belimumab than placebo, as were sustained responses for SRI4 (belimumab, 43.4%; placebo, 41.0%; OR 1.08 (95% CI 0.46 to 2.52)) and Parent-global (belimumab, 59.1%; placebo, 33.3%; OR 3.49 (95% CI 1.23 to 9.91)). Serious adverse events were reported in 17.0% of belimumab patients and 35.0% of placebo patients; one death occurred (placebo). Week-52, geometric mean (95% CI) belimumab trough concentration was 56.2 (45.2 to 69.8) µg/mL.
Conclusion: The belimumab intravenous pharmacokinetics and benefit-risk profile in cSLE are consistent with adult belimumab studies and the 10 mg/kg every 4 weeks dose is appropriate.
Trial registration number: NCT01649765.
Keywords: DMARDs (biologic); systemic lupus erythematosus; treatment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: DB, KC, AH, AN, DAR, BJ and HS are employees of GSK and hold shares/options in the company; M-LW is a former employee of GSK; HIB has served the speakers bureau of GSK, Roche and Novartis, has been a consultant to Hoffman-La Roche, Novartis, Pfizer, Sanofi Aventis, Merck Serono, AbbVie, Amgen, Alter, AstraZeneca, Baxalta Biosimilars, Biogen Idec, Boehringer, BMS, Celgene, EMD Serono, Janssen, MedImmune, Novartis, Pfizer and UCB Biosciences GmbH. Payments are made to CCHMC, the employer of HIB; AM has received honoraria for consultancies (<10 000 US$ each) from Eli-Lilly, EMD Serono, Janssen, Novartis, Pfizer and AbbVie; DML has received honoraria and/or consulting fees (<10 000 US$ each) from AbbVie, Janssen and Sobi; NR has received speaker’s bureau and reimbursement of travel expenses from GSK, honoraria for consultancies (<10 000 US$ each) from Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer, Bristol Myers Squibb, Eli-Lilly, EMD Serono, GSK, Hoffmann-La Roche, Janssen, Merck, Novartis, Pfizer, R-Pharma, Sanofi, Servier, Sinergie, Sobi and Takeda. The IRCCS Istituto Giannina Gaslini (IGG), where NR works as full-time public employee has received contributions (>10 000 US$ each) from BMS, Eli-Lilly, GSK, Hoffmann-La Roche, Janssen, Novartis, Pfizer and Sobi. This funding has been reinvested for research activities of the hospital in a fully independent manner, without any commitment with third parties.; JA has received consulting fees and/or honoraria from AbbVie, BMS, Gebro, Novartis, Pfizer, Roche and Sobi; CA-M has received honoraria for consultancies or speaker bureaus from Pfizer, Eli Lilly and Takeda; ICP has received consulting fees from AbbVie, BMS, Novartis, Pfizer, Roche and Sobi; MS has received honoraria for consultancies or speaker bureaus (<10 000 USD each) from AbbVie, Medac Pharm and Novartis.
Figures