Randomised, cOntrolled Multicentre trial of 26 weeks subcutaneous liraglutide (a glucagon-like peptide-1 receptor Agonist), with or without contiNuous positive airway pressure (CPAP), in patients with type 2 diabetes mellitus (T2DM) and obstructive sleep apnoEa (OSA) (ROMANCE): study protocol assessing the effects of weight loss on the apnea-hypnoea index (AHI)
- PMID: 32699168
- PMCID: PMC7380950
- DOI: 10.1136/bmjopen-2020-038856
Randomised, cOntrolled Multicentre trial of 26 weeks subcutaneous liraglutide (a glucagon-like peptide-1 receptor Agonist), with or without contiNuous positive airway pressure (CPAP), in patients with type 2 diabetes mellitus (T2DM) and obstructive sleep apnoEa (OSA) (ROMANCE): study protocol assessing the effects of weight loss on the apnea-hypnoea index (AHI)
Abstract
Introduction: Obstructive sleep apnoea (OSA) and type 2 diabetes mellitus (T2DM) often occur concurrently, and untreated OSA may potentially amplify the high risk of cardiovascular disease in T2DM. Compliance with continuous positive airway pressure (CPAP), the conventional treatment for OSA, can be poor and considering weight loss is the most effective treatment for OSA. This trial examines whether the glucagon-like peptide-1 receptor agonist liraglutide, a glucose-lowering therapy associated with significant weight loss used in T2DM, can improve the severity and symptoms of OSA.
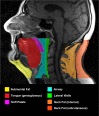
Methods and analysis: This is an outpatient, single-centred, open-labelled, prospective, phase IV randomised controlled trial in a two-by-two factorial design. One hundred and thirty-two patients with newly diagnosed OSA (apnoea-hypopnoea index (AHI) ≥15 events/hour), and existing obesity and T2DM (glycated haemoglobin (HbA1c) ≥47 mmol/mol), will be recruited from diabetes and sleep medicine outpatient clinics in primary and secondary care settings across Liverpool. Patients will be allocated equally, using computer-generated random, permuted blocks of unequal sizes, to each of the four treatment arms for 26 weeks: (i) liraglutide (1.8 mg once per day) alone, (ii) liraglutide 1.8 mg once per day with CPAP, (iii) CPAP alone (conventional care) or (iv) no treatment (control). The primary outcome measure is change in OSA severity, determined by AHI. Secondary outcome measures include effects on glycaemic control (glycated haemoglobin (HbA1c)), body weight and quality of life measures. Exploratory measures include measures of physical activity, MRI-derived measures of regional body composition including fat mass (abdominal subcutaneous, visceral, neck and liver fat) and skeletal muscle mass (cross-sectional analysis of thigh), indices of cardiac function (using transthoracic echocardiography) and endothelial function.
Ethical approval: The study has been approved by the North West Liverpool Central Research Ethics Committee (14/NW/1019) and it is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Trial registration numbers: ISRCTN16250774. EUDRACT No. 2014-000988-41. UTN U1111-1139-0677.
Keywords: diabetes & endocrinology; protocols & guidelines; sleep medicine.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DJC has competing interests with AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals and Lilly & Novo Nordisk. JW has acted as a consultant, received institutional grants and given lectures on behalf of pharmaceutical companies developing or marketing medicines used for the treatment of diabetes, specifically AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Lilly, Novo Nordisk and Sanofi & Takeda.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
