Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma
- PMID: 32699265
- PMCID: PMC8026986
- DOI: 10.1038/s41401-020-0478-3
Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma
Abstract
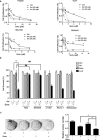
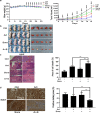
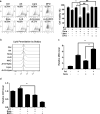
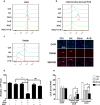
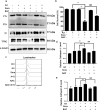
Sorafenib is the first-line medication for advanced hepatocellular carcinoma (HCC), but it can only extend limited survival. It is imperative to find a combination strategy to increase sorafenib efficacy. Artesunate is such a preferred candidate, because artesunate is clinically well-tolerated and more importantly both drugs can induce ferroptosis through different mechanisms. In this study we investigated the combined effect of sorafenib and artesunate in inducing ferroptosis of HCC and elucidated the involved molecular mechanisms. We showed that artesunate greatly enhanced the anticancer effects of low dose of sorafenib against Huh7, SNU-449, and SNU-182 HCC cell lines in vitro and against Huh7 cell xenograft model in Balb/c nude mice. The combination index method confirmed that the combined effect of sorafenib and artesunate was synergistic. Compared with the treatment with artesunate or sorafenib alone, combined treatment induced significantly exacerbated lipid peroxidation and ferroptosis, which was blocked by N-acetyl cysteine and ferroptosis inhibitors liproxstatin-1 and deferoxamine mesylate, but not by inhibitors of other types of cell death (z-VAD, necrostatin-1 and belnacasan). In Huh7 cells, we demonstrated that the combined treatment induced oxidative stress and lysosome-mediated ferritinophagy, two essential aspects of ferroptosis. Sorafenib at low dose mainly caused oxidative stress through mitochondrial impairments and SLC7A11-invovled glutathione depletion. Artesunate-induced lysosome activation synergized with sorafenib-mediated pro-oxidative effects by promoting sequential reactions including lysosomal cathepsin B/L activation, ferritin degradation, lipid peroxidation, and consequent ferroptosis. Taken together, artesunate could be repurposed to sensitize sorafenib in HCC treatment. The combined treatment can be easily translated into clinical applications.
Keywords: Artesunate; Ferroptosis; HCC; Lysosome; Mitochondria; Sorafenib.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

