Vitamin D protects against immobilization-induced muscle atrophy via neural crest-derived cells in mice
- PMID: 32699341
- PMCID: PMC7376070
- DOI: 10.1038/s41598-020-69021-y
Vitamin D protects against immobilization-induced muscle atrophy via neural crest-derived cells in mice
Abstract
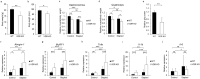
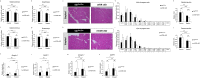
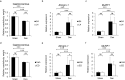
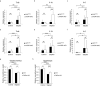
Vitamin D deficiency is a recognized risk factor for sarcopenia development, but mechanisms underlying this outcome are unclear. Here, we show that low vitamin D status worsens immobilization-induced muscle atrophy in mice. Mice globally lacking vitamin D receptor (VDR) exhibited more severe muscle atrophy following limb immobilization than controls. Moreover, immobilization-induced muscle atrophy was worse in neural crest-specific than in skeletal muscle-specific VDR-deficient mice. Tnfα expression was significantly higher in immobilized muscle of VDR-deficient relative to control mice, and was significantly elevated in neural crest-specific but not muscle-specific VDR-deficient mice. Furthermore, muscle atrophy induced by limb immobilization in low vitamin D mice was significantly inhibited in Tnfα-deficient mice. We conclude that vitamin D antagonizes immobilization-induced muscle atrophy via VDR expressed in neural crest-derived cells.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Conzade R, et al. Vitamin D in relation to incident sarcopenia and changes in muscle parameters among older adults: the KORA-age study. Calcif. Tissue Int. 2019;105:173–182. - PubMed
-
- Scott D, Blizzard L, Fell J, Ding C, Winzenberg T, Jones G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin. Endocrinol. 2010;73:581–587. - PubMed
-
- Cummings SR, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. - PubMed
-
- Odvina CV, Wergedal JE, Libanati CR, Schulz EE, Baylink DJ. Relationship between trabecular vertebral body density and fractures: a quantitative definition of spinal osteoporosis. Metabolism. 1988;37:221–228. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

