Bone mineral density and mortality in end-stage renal disease patients
- PMID: 32699616
- PMCID: PMC7367137
- DOI: 10.1093/ckj/sfaa089
Bone mineral density and mortality in end-stage renal disease patients
Abstract
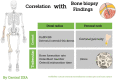
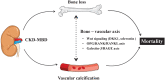
Osteoporosis characterized by low bone mineral density (BMD) as assessed by dual-energy X-ray absorptiometry (DXA) is common among end-stage renal disease (ESRD) patients and associates with high fracture incidence and high all-cause mortality. This is because chronic kidney disease-mineral bone disorders (CKD-MBDs) promote not only bone disease (osteoporosis and renal dystrophy) but also vascular calcification and cardiovascular disease. The disturbed bone metabolism in ESRD leads to 'loss of cortical bone' with increased cortical porosity and thinning of cortical bone rather than to loss of trabecular bone. Low BMD, especially at cortical-rich bone sites, is closely linked to CKD-MBD, vascular calcification and poor cardiovascular outcomes. These effects appear to be largely mediated by shared mechanistic pathways via the 'bone-vascular axis' through which impaired bone status associates with changes in the vascular wall. Thus, bone is more than just the scaffolding that holds the body together and protects organs from external forces but is-in addition to its physical supportive function-also an active endocrine organ that interacts with the vasculature by paracrine and endocrine factors through pathways including Wnt signalling, osteoprotegerin (OPG)/receptor activator of nuclear factor-κB (RANK)/RANK ligand system and the Galectin-3/receptor of advanced glycation end products axis. The insight that osteogenesis and vascular calcification share many similarities-and the knowledge that vascular calcification is a cell-mediated active rather than a passive mineralization process-suggest that low BMD and vascular calcification ('vascular ossification') to a large extent represent two sides of the same coin. Here, we briefly review changes of BMD in ESRD as observed using different DXA methods (central and whole-body DXA) at different bone sites for BMD measurements, and summarize recent knowledge regarding the relationships between 'low BMD' and 'fracture incidence, vascular calcification and increased mortality' in ESRD patients, as well as potential 'molecular mechanisms' underlying these associations.
Keywords: bone mineral density; bone–vascular axis; end-stage renal disease; mortality; osteoporosis; vascular calcification.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures


References
-
- Kooman JP, Kotanko P, Schols A. et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014; 10: 732–742 - PubMed
-
- Briggs AM, Perilli E, Parkinson IH. et al. Measurement of subregional vertebral bone mineral density in vitro using lateral projection dual-energy X-ray absorptiometry: validation with peripheral quantitative computed tomography. J Bone Miner Metab 2012; 30: 222–231 - PubMed
-
- Matsuoka M, Iseki K, Tamashiro M. et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol 2004; 8: 54–58 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

