The ANTENATAL multicentre study to predict postnatal renal outcome in fetuses with posterior urethral valves: objectives and design
- PMID: 32699617
- PMCID: PMC7367108
- DOI: 10.1093/ckj/sfz107
The ANTENATAL multicentre study to predict postnatal renal outcome in fetuses with posterior urethral valves: objectives and design
Abstract
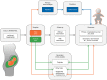
Background: Posterior urethral valves (PUV) account for 17% of paediatric end-stage renal disease. A major issue in the management of PUV is prenatal prediction of postnatal renal function. Fetal ultrasound and fetal urine biochemistry are currently employed for this prediction, but clearly lack precision. We previously developed a fetal urine peptide signature that predicted in utero with high precision postnatal renal function in fetuses with PUV. We describe here the objectives and design of the prospective international multicentre ANTENATAL (multicentre validation of a fetal urine peptidome-based classifier to predict postnatal renal function in posterior urethral valves) study, set up to validate this fetal urine peptide signature.
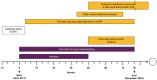
Methods: Participants will be PUV pregnancies enrolled from 2017 to 2021 and followed up until 2023 in >30 European centres endorsed and supported by European reference networks for rare urological disorders (ERN eUROGEN) and rare kidney diseases (ERN ERKNet). The endpoint will be renal/patient survival at 2 years postnatally. Assuming α = 0.05, 1-β = 0.8 and a mean prevalence of severe renal outcome in PUV individuals of 0.35, 400 patients need to be enrolled to validate the previously reported sensitivity and specificity of the peptide signature.
Results: In this largest multicentre study of antenatally detected PUV, we anticipate bringing a novel tool to the clinic. Based on urinary peptides and potentially amended in the future with additional omics traits, this tool will be able to precisely quantify postnatal renal survival in PUV pregnancies. The main limitation of the employed approach is the need for specialized equipment.
Conclusions: Accurate risk assessment in the prenatal period should strongly improve the management of fetuses with PUV.
Keywords: development; kidney disease; obstructive uropathy; prediction; prenatal biomarkers.
© The Author(s) 2019. Published by Oxford University Press on behalf of ERA-EDTA.
Figures

References
-
- Thakkar D, Deshpande AV, Kennedy SE. Epidemiology and demography of recently diagnosed cases of posterior urethral valves. Pediatr Res 2014; 76: 560–563 - PubMed
-
- Agarwal S. Urethral valves. BJU Int 1999; 84: 570–578 - PubMed
-
- Krishnan A, de Souza A, Konijeti R, et al. The anatomy and embryology of posterior urethral valves. J Urol 2006; 175: 1214–1220 - PubMed
-
- Morris RK, Ruano R, Kilby MD. Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol 2011; 37: 629–637 - PubMed

