Artificial Signal Transduction
- PMID: 32699734
- PMCID: PMC7271652
- DOI: 10.1002/open.201900367
Artificial Signal Transduction
Abstract
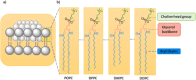
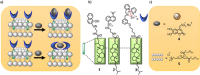
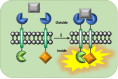
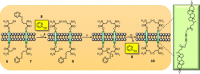
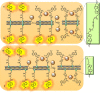
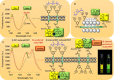
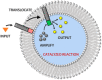
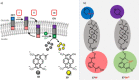
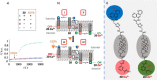
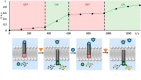
Communication between and inside cells as well as their response to external stimuli relies on elaborated systems of signal transduction. They all require a directional transmission across membranes, often realized by primary messenger docking onto external receptor units and subsequent internalization of the signal in form of a released second messenger. This in turn starts a cascade of events which ultimately control all functions of the living cell. Although signal transduction is a fundamental biological process realized by supramolecular recognition and multiplication events with small molecules, chemists have just begun to invent artificial models which allow to study the underlying rules, and one day perhaps to rescue damaged transduction systems in nature. This review summarizes the exciting pioneering efforts of chemists to create simple models for the basic principles of signal transduction across a membrane. It starts with first attempts to establish molecular recognition events on liposomes with embedded receptor amphiphiles and moves on to simple transmembrane signaling across lipid bilayers. More elaborated systems step by step incorporate more elements of cell signaling, such as primary and secondary messenger or a useful cellular response such as cargo release.
Keywords: cell membranes; fluorescence; liposomes; messengers; signal transduction.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

