Non-steroidal anti-inflammatory drugs, prostaglandins, and COVID-19
- PMID: 32700336
- PMCID: PMC7405053
- DOI: 10.1111/bph.15206
Non-steroidal anti-inflammatory drugs, prostaglandins, and COVID-19
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the novel coronavirus disease 2019 (COVID-19), a highly pathogenic and sometimes fatal respiratory disease responsible for the current 2020 global pandemic. Presently, there remains no effective vaccine or efficient treatment strategies against COVID-19. Non-steroidal anti-inflammatory drugs (NSAIDs) are medicines very widely used to alleviate fever, pain, and inflammation (common symptoms of COVID-19 patients) through effectively blocking production of prostaglandins (PGs) via inhibition of cyclooxyganase enzymes. PGs can exert either proinflammatory or anti-inflammatory effects depending on the inflammatory scenario. In this review, we survey the potential roles that NSAIDs and PGs may play during SARS-CoV-2 infection and the development and progression of COVID-19. LINKED ARTICLES: This article is part of a themed issue on The Pharmacology of COVID-19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc.
Keywords: COVID-19; NSAID; SARS-CoV-2; cytokine storm; inflammation; prostaglandin.
© 2020 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

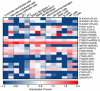
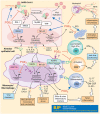

References
-
- Aksoy, M. O. , Li, X. , Borenstein, M. , Yi, Y. , & Kelsen, S. G. (1999). Effects of topical corticosteroids on inflammatory mediator‐induced eicosanoid release by human airway epithelial cells. The Journal of Allergy and Clinical Immunology, 103, 1081–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

