Consistent improvement with eculizumab across muscle groups in myasthenia gravis
- PMID: 32700461
- PMCID: PMC7448154
- DOI: 10.1002/acn3.51121
Consistent improvement with eculizumab across muscle groups in myasthenia gravis
Abstract
Objective: To assess whether eculizumab, a terminal complement inhibitor, improves patient- and physician-reported outcomes (evaluated using the myasthenia gravis activities of daily living profile and the quantitative myasthenia gravis scale, respectively) in patients with refractory anti-acetylcholine receptor antibody-positive generalized myasthenia gravis across four domains, representing ocular, bulbar, respiratory, and limb/gross motor muscle groups.
Methods: Patients with refractory anti-acetylcholine receptor antibody-positive generalized myasthenia gravis were randomized 1:1 to receive either placebo or eculizumab during the REGAIN study (NCT01997229). Patients who completed REGAIN were eligible to continue into the open-label extension trial (NCT02301624) for up to 4 years. The four domain scores of each of the myasthenia gravis activities of daily living profile and the quantitative myasthenia gravis scale recorded throughout REGAIN and through 130 weeks of the open-label extension were analyzed.
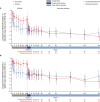
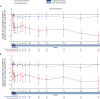
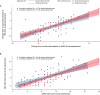
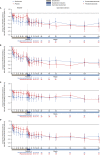
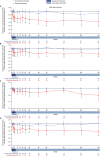
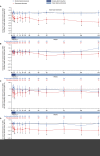
Results: Of the 125 patients who participated in REGAIN, 117 enrolled in the open-label extension; 61 had received placebo and 56 had received eculizumab during REGAIN. Patients experienced rapid improvements in total scores and all four domain scores of both the myasthenia gravis activities of daily living profile and the quantitative myasthenia gravis scale with eculizumab treatment. These improvements were sustained through 130 weeks of the open-label extension.
Interpretation: Eculizumab treatment elicits rapid and sustained improvements in muscle strength across ocular, bulbar, respiratory, and limb/gross motor muscle groups and in associated daily activities in patients with refractory anti-acetylcholine receptor antibody-positive generalized myasthenia gravis.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
R.M. and J.F.H. have received research support, honoraria, and nonfinancial support from Alexion Pharmaceuticals, which owns patent rights to eculizumab that was used in this study. F.L.O'B. and M.Y. are employed by, and own stock in, Alexion Pharmaceuticals, which owns patent rights to eculizumab that was used in this study.
Figures

References
-
- Engel‐Nitz NM, Boscoe AN, Wolbeck R, et al. Burden of illness in patients with treatment refractory myasthenia gravis. Muscle Nerve 2018;58:99–105. - PubMed
-
- Lindstrom JM, Seybold ME, Lennon VA, et al. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 1976;26:1054–1059. - PubMed
-
- Mantegazza R, Pareyson D, Baggi F, et al. Anti AChR antibody: relevance to diagnosis and clinical aspects of myasthenia gravis. Ital J Neurol Sci 1988;9:141–145. - PubMed
-
- Oh SJ, Kim DE, Kuruoglu R, et al. Diagnostic sensitivity of the laboratory tests in myasthenia gravis. Muscle Nerve 1992;15:720–724. - PubMed

