Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment
- PMID: 32700630
- PMCID: PMC7792231
- DOI: 10.1161/JAHA.120.017293
Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment
Abstract
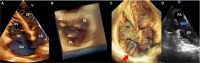
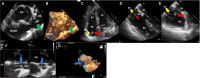
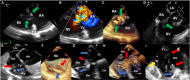
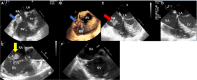
Compared with the extensive data on left-sided infective endocarditis (IE), there is much less published information on the features and management of right-sided IE. Right-sided IE accounts for 5% to 10% of all IE cases, and compared with left-sided IE, it is more often associated with intravenous drug use, intracardiac devices, and central venous catheters, all of which has become more prevalent over the past 20 years. In this manuscript on right-sided IE we provide an up-to-date overview on the epidemiology, etiology, microbiology, potential locations of infection in the right heart, diagnosis, imaging, common complications, management, and prognosis. We present updated information on the treatment of pacemaker and device infections, infected fibrin sheaths that appear to be an easily missed source of infection after central line as well as pacemaker removal. We review current data on the AngioVac percutaneous aspiration device, which can obviate the need for surgery in patients with infected pacemaker leads and fibrin sheaths. We also focused on advanced diagnostic modalities, such as positron emission tomography/computed tomography. All of these are supported by specific case examples with detailed echocardiographic imaging from our experience.
Keywords: echocardiography; infective endocarditis; right‐sided infective endocarditis; tricuspid.
Figures
References
-
- Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–893. - PubMed
-
- Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65:2070–2076. - PubMed
-
- Sambola A, Fernandez‐Hidalgo N, Almirante B, Roca I, Gonzalez‐Alujas T, Serra B, Pahissa A, Garcia‐Dorado D, Tornos P. Sex differences in native‐valve infective endocarditis in a single tertiary‐care hospital. Am J Cardiol. 2010;106:92–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

