GLP-1 receptor agonists for Parkinson's disease
- PMID: 32700772
- PMCID: PMC7390475
- DOI: 10.1002/14651858.CD012990.pub2
GLP-1 receptor agonists for Parkinson's disease
Abstract
Background: Parkinson's disease (PD) is a progressive disorder characterised by both motor and non-motor problems. Glucagon-like peptide-1 (GLP-1) receptor agonists, licensed for treatment of type 2 diabetes, work by stimulating GLP-1 receptors in the pancreas, which triggers the release of insulin. GLP-1 receptors have been found in the brain. Insulin signalling in the brain plays a key role in neuronal metabolism and repair and in synaptic efficacy, but insulin signalling is desensitised in the brain of people with PD. Researchers are exploring the neuroprotective effects of GLP-1 receptor agonists in neurodegenerative disorders such as PD.
Objectives: To evaluate the effectiveness and safety of GLP-1 receptor agonists for Parkinson's disease.
Search methods: We searched the Cochrane Movement Disorders Group trials register; the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; and Ovid MEDLINE and Embase. We also searched clinical trials registries, and we handsearched conference abstracts. The most recent search was run on 25 June 2020.
Selection criteria: We included randomised controlled trials (RCTs) of adults with PD that compared GLP-1 receptor agonists with conventional PD treatment, placebo, or no treatment.
Data collection and analysis: Two review authors independently assessed studies for inclusion, extracted data, and assessed risk of bias. We rated the quality of evidence using GRADE. We resolved discrepancies between the two data extractors by consultation with a third review author.
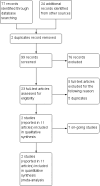
Main results: Through our searches, we retrieved 99 unique records, of which two met our inclusion criteria. One double-blind study of exenatide versus placebo randomised 62 participants, who self-administered exenatide or placebo for 48 weeks and were followed up at 60 weeks after a 12-week washout. One single-blind study of exenatide versus no additional treatment randomised 45 participants; participants in the intervention group self-administered exenatide for 12 months, and all participants were followed up at 14 months and 24 months following absence of exenatide for 2 months and 12 months, respectively. These trials had low risk of bias, except risk of performance bias was high for Aviles-Olmos 2013. Exenatide versus placebo Primary outcomes We found low-certainty evidence suggesting that exenatide improves motor impairment as assessed by the Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Part III in the off-medication state (mean difference (MD) -3.10, 95% confidence interval (CI) -6.11 to -0.09). The difference in scores was slightly greater when scores were adjusted for baseline severity of the condition (as reported by study authors) (MD -3.5, 95% CI -6.7 to -0.3), exceeding the minimum clinically important difference (MCID). We found low-certainty evidence suggesting that exenatide has little or no effect on health-related quality of life (HRQoL) as assessed by the Parkinson's Disease Questionnaire (PDQ)-39 Summary Index (SI) (MD -1.80, 95% CI -6.95 to 3.35), the EuroQol scale measuring health status in five dimensions (EQ5D) (MD 0.07, 95% CI -0.03 to 0.16), or the EQ5D visual analogue scale (VAS) (MD 5.00, 95% CI -3.42 to 13.42). Eight serious adverse events (SAEs) were recorded, but all were considered unrelated to the intervention. Low-certainty evidence suggests that exenatide has little or no effect on weight loss (risk ratio (RR) 1.25, 95% CI 0.89 to 1.76). Exenatide versus no treatment Primary outcomes at 14 months We found very low-certainty evidence suggesting that exenatide improves motor impairment as assessed by MDS-UPDRS Part III off medication (MD -4.50, 95% CI -8.64 to -0.36), exceeding the MCID. We are uncertain whether exenatide improves HRQoL as assessed by the PDQ-39 SI (MD 3.50, 95% CI -2.75 to 9.75; very low-quality evidence). We found very low-certainty evidence suggesting that exenatide has little or no effect on the number of SAEs (RR 1.60, 95% 0.40 to 6.32). We found very low-certainty evidence suggesting that exenatide may lead to weight loss (MD -2.40 kg, 95% CI -4.56 to -0.24). Primary outcomes at 24 months We found evidence as reported by study authors to suggest that exenatide improves motor impairment as measured by MDS-UPDRS Part III off medication (MD 5.6 points, 95% CI 2.2 to 9.0). Exenatide may not improve HRQoL as assessed by the PDQ-39 SI (P = 0.682) and may not result in weight loss (MD 0.1 kg, 95% CI 3.0 to 2.8).
Authors' conclusions: Low- or very low-certainty evidence suggests that exenatide may improve motor impairment for people with PD. The difference in motor impairment observed between groups may persist for some time following cessation of exenatide. This raises the possibility that exenatide may have a disease-modifying effect. SAEs were unlikely to be related to treatment. The effectiveness of exenatide for improving HRQoL, non-motor outcomes, ADLs, and psychological outcomes is unclear. Ongoing studies are assessing other GLP-1 receptor agonists.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
CAM: none known.
GSD: none known.
JH: none known.
DE: none known.
SM: none known.
RW: none known.
HCAE: none known.
Figures
























Update of
- doi: 10.1002/14651858.CD012990
References
References to studies included in this review
Athauda 2017 {published data only}
-
- Athauda D, Budnik N, Chowdhury K, Skene S, Foltynie T. The effect of exenatide on specific non-motor symptoms in Parkinson’s disease – a post-hoc analysis. In: European Journal of Neurology. Issue S2 edition. Vol. 25. Abstracts of the 4th Congress of the European Academy of Neurology, Lisbon, Portugal, June 2018, 2018:309.
Aviles‐Olmos 2013 {published data only}
-
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. Exenatide and motor symptoms in Parkinson's disease (PD) [abstract]. In: Movement Disorders. Vol. 29 Suppl 1:611. 2014.
-
- Aviles-Olmos I, Dickson J, Kefalopoupou Z, Djamshidian A, Kahan J, Ell P, et al. Exenatide and non motor symptoms In Parkinson's disease (PD) [abstract]. In: Movement Disorders. Vol. 29 Suppl 1 6:12. 2014.
-
- Aviles-Olmos I, Kefalopoulou Z, Djamshidian A, Limousin P, Dickson J, Lees A, et al. An open label, single site, 12 month, phase II, randomised controlled trial evaluating the safety and efficacy of Exendin-4 (exenatide) in the treatment of patients with moderate severity Parkinson's disease [abstract]. Movement Disorders 2012;27(Suppl 1:343).
References to ongoing studies
Clinicaltrials.gov identifier: NCT02953665 {published data only}
-
- A phase II, randomized, double-blinded, placebo-controlled trial of liraglutide in Parkinson’s disease. Ongoing study. April 2017. Contact author for more information.
ClinicalTrials.gov Identifier: NCT03439943 {published data only}
-
- Study to evaluate the effect of lixisenatide in patients with Parkinson's disease (LixiPark). Ongoing study. June 2018. Contact author for more information.
ClinicalTrials.gov Identifier: NCT03659682 {published data only}
-
- Effect of GLP1R stimulation on neuroprotection and inflammation in Parkinson's disease. Ongoing study. January 2019. Contact author for more information.
Clinicaltrials.gov identifier: NCT04154072 {published data only}
-
- Multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of 36 weeks of treatment with NLY01 in early-stage Parkinson’s disease. Ongoing study. February 2020. Contact author for more information.
ClinicalTrials.gov Identifier: NCT04232969 {published data only}
-
- Exenatide once weekly over 2 years as a potential disease modifying treatment for Parkinson's disease (Exenatide-PD3). Ongoing study. January 2020. Contact author for more information.
Clinicaltrials.gov identifier: NCT04269642 {published data only}
-
- Effects of exenatide on motor function and the brain. Ongoing study. March 2020. Contact author for more information.
ClinicalTrials.gov Identifier: NCT04305002 {published data only}
-
- Effect of exenatide on disease progression in early Parkinson’s disease. Ongoing study. January 2020. Contact author for more information.
Additional references
Abou‐Sleiman 2006
Al‐Bachari 2017
Ascherio 2016
Aviles‐Olmos 2013a
Aviles‐Olmos 2013b
Aviles‐Olmos 2014
Baggio 2007
Bomfim 2012
-
- Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated A-beta oligomers. Journal of Clinical Investigation 2012;122:1339-53. [DOI: 10.1172/JCI57256] - DOI - PMC - PubMed
Campbell 2013
Chaudhuri 2008
Collins 2012
de Boer 1996
de Lau 2006
Doyle 2003
Egger 1997
Erbil 2019
Fahn 1987
-
- Fahn S, Elton R, Members of the UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors(s). Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park (NJ): Macmillan Health Care Information, 1987:153-163, 293-304.
Foltynie 2020 [pers comm]
-
- Foltynie T. Random sequence generation and allocation concealment used in our trial [personal communication]. Email to: C Mulvaney. 4 May 2020.
Freiherr 2013
Ghasemi 2013
Goetz 2000
Goetz 2008
GRADEpro 2015 [Computer program]
-
- GRADEpro GDT. Version accessed 18 October 2017. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Gray 2015
Hammarlund 2012
Harkavyi 2008
Hely 2005
Hely 2008
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirsch 2016
Hoehn 1967
Holst 2004
Horváth 2015
-
- Horváth K, Ascherman Z, Ács P, Deli G, Janszky J, Komoly S, et al. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism & Related Disorders 2015;21(12):1421-6. [10.1016/j.parkreldis.2015.10.006] - PubMed
Horváth 2017
Hu 2007
Hughes 1992
Hölscher 2014
Hölscher 2018
Jalewa 2016
Jenkinson 1997
-
- Jenkinson C, Fitzpatrick R, Peto V, Grenhall R, Hyman N. The PDQ-8: development and validation of a short-form Parkinson’s disease questionnaire. Psychology and Health 1997;12:805-14. [DOI: 10.1002/mds.10678] - DOI
Ji 2016
Kalia 2015
Li 2009
-
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proceedings of the National Academy of Sciences of the United States of America 2009;106:1285-90. [DOI: 10.1073/pnas.0806720106] - DOI - PMC - PubMed
Li 2010
-
- Li Y, Duffy K, Ottinger M, Ray B, Bailey J, Holloway H, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. Journal of Alzheimer's Disease 2010;19(4):1205-19. [DOI: 10.3233/JAD-2010-1314] - DOI - PMC - PubMed
Li 2016
Liberati 2009
Liu 2015a
Liu 2015b
Long‐Smith 2013
-
- Long-Smith CM, Manning S, McClean PL, Coakley MF, O'Halloran DJ, Holscher C, et al. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-beta plaque and glial pathology in a mouse model of Alzheimer's disease. Neuromolecular Medicine 2013;15:102-14. [DOI: 10.1007/s12017-012-8199-5] - DOI - PubMed
Malek 2016
Mattis 1976
-
- Mattis S. Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. New York (NY): Grune and Stratton Inc, 1976.
McClean 2011
McGhee 2013
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [PMID: ] - PubMed
Moroo 1994
-
- Moroo I, Yamada T, Makino H, Tooyama I, McGeer PL, McGeer EG, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson's disease. Acta Neuropathologica 1994;87:343-8. [PMID: ] - PubMed
Morris 2011
Müller 2000
-
- Müller T, Woitalla D, Saft C, Kuhn W. Levodopa in plasma correlates with body weight of parkinsonian patients. Parkinsonism & Related Disorders 2000;6(3):171-3. [PMID: PMID: 10817957] - PubMed
Peto 1995
-
- Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Quality of Life Research 1995;4:241-8. [PMID: ] - PubMed
Pleuvry 2004
-
- Pleuvry BJ. Receptors, agonists and antagonists. Anaesthesia & Intensive Care Medicine 2004;5(10):350-2. [DOI: ]
Pringhseim 2014
RevMan 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schernhammer 2011
Schwab 1969
-
- Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson MC, editors(s). Third Symposium on Parkinson’s Disease. Edinburgh & London (UK): E & S Livingstone, 1969:152-7.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sun 2012
van der Heide 2006
Vilsbøll 2012
Wahlqvist 2012
-
- Wahlqvist ML, Lee MS, Hsu CC, Chuang SY, Lee JT, Tsai HN. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson's disease occurring with type 2 diabetes in a Taiwanese population cohort. Parkinsonism & Related Disorders 2012;18:753-8. [DOI: 10.1016/j.parkreldis.2012.03.010] - DOI - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

