Light-Activated Carbon Monoxide Prodrugs Based on Bipyridyl Dicarbonyl Ruthenium(II) Complexes
- PMID: 32700815
- PMCID: PMC7496190
- DOI: 10.1002/chem.202002139
Light-Activated Carbon Monoxide Prodrugs Based on Bipyridyl Dicarbonyl Ruthenium(II) Complexes
Abstract
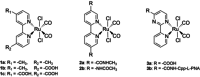
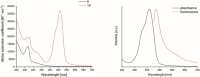
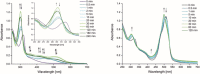
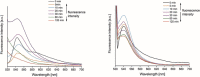
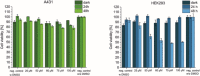
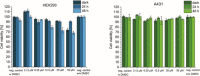
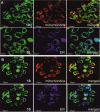
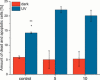
Two photoactivatable dicarbonyl ruthenium(II) complexes based on an amide-functionalised bipyridine scaffold (4-position) equipped with an alkyne functionality or a green-fluorescent BODIPY (boron-dipyrromethene) dye have been prepared and used to investigate their light-induced decarbonylation. UV/Vis, FTIR and 13 C NMR spectroscopies as well as gas chromatography and multivariate curve resolution alternating least-squares analysis (MCR-ALS) were used to elucidate the mechanism of the decarbonylation process. Release of the first CO molecule occurs very quickly, while release of the second CO molecule proceeds more slowly. In vitro studies using two cell lines A431 (human squamous carcinoma) and HEK293 (human embryonic kidney cells) have been carried out in order to characterise the anti-proliferative and anti-apoptotic activities. The BODIPY-labelled compound allows for monitoring the cellular uptake, showing fast internalisation kinetics and accumulation at the endoplasmic reticulum and mitochondria.
Keywords: anti-apoptotic activity; anti-proliferative; cellular localisation; photoCORM; ruthenium(II).
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
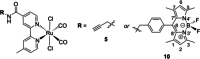

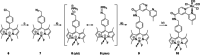


References
-
- None
-
- Mann B. E., Organometallics 2012, 31, 5728–5735;
-
- Motterlini R., Otterbein L. E., Nat. Rev. Drug Discovery 2010, 9, 728–743; - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

