Efficacy and Safety of Locoregional Radiotherapy With Chemotherapy vs Chemotherapy Alone in De Novo Metastatic Nasopharyngeal Carcinoma: A Multicenter Phase 3 Randomized Clinical Trial
- PMID: 32701129
- PMCID: PMC7378870
- DOI: 10.1001/jamaoncol.2020.1808
Efficacy and Safety of Locoregional Radiotherapy With Chemotherapy vs Chemotherapy Alone in De Novo Metastatic Nasopharyngeal Carcinoma: A Multicenter Phase 3 Randomized Clinical Trial
Abstract
Importance: The role of locoregional radiotherapy in patients with de novo metastatic nasopharyngeal carcinoma (mNPC) is unclear.
Objective: To investigate the efficacy and safety of locoregional radiotherapy in de novo mNPC.
Design, setting, and participants: Patients with biopsy-proven mNPC, who demonstrated complete or partial response (RECIST v1.1) following 3 cycles of cisplatin and fluorouracil chemotherapy, were enrolled. Eligible patients were randomly assigned (1:1) to receive either chemotherapy plus radiotherapy or chemotherapy alone. Overall, 126 of 173 patients screened were eligible to the study, and randomized to chemotherapy plus radiotherapy (n = 63) or chemotherapy alone (n = 63). Median (IQR) follow-up duration was 26.7 (17.2-33.5) months.
Interventions: The chemotherapy regimens were fluorouracil continuous intravenous infusion at 5 g/m2 over 120 hours and 100 mg/m2 intravenous cisplatin on day 1, administered every 3 weeks for 6 cycles. Patients assigned to the chemotherapy plus radiotherapy group received intensity-modulated radiotherapy (IMRT) after chemotherapy.
Main outcomes and measures: The primary end point of the study was overall survival (OS). The secondary end point was progression-free survival (PFS) and safety.
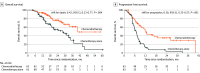
Results: Overall, 126 patients were enrolled (105 men [83.3%] and 21 women [16.7%]; median [IQR] age, 46 [39-52] years). The 24-month OS was 76.4% (95% CI, 64.4%-88.4%) in the chemotherapy plus radiotherapy group, compared with 54.5% (95% CI, 41.0%-68.0%) in the chemotherapy-alone group. The study met its primary end point of improved OS (stratified hazard ratio [HR], 0.42; 95% CI, 0.23-0.77; P = .004) in favor of chemotherapy plus radiotherapy. Progression-free survival was also improved in the chemotherapy plus radiotherapy group compared with the chemotherapy-alone group (stratified HR, 0.36; 95% CI, 0.23-0.57). No significant differences in acute hematological or gastrointestinal toxic effects were observed between the treatment arms. The frequency of acute grade 3 or higher dermatitis, mucositis, and xerostomia was 8.1%, 33.9%, and 6.5%, respectively, in the chemotherapy plus radiotherapy group. The frequency of late severe grade 3 or higher hearing loss and trismus was 5.2% and 3.4%, respectively, in the chemotherapy plus radiotherapy group.
Conclusions and relevance: In this randomized clinical trial, radiotherapy added to chemotherapy significantly improved OS in chemotherapy-sensitive patients with mNPC.
Trial registration: ClinicalTrials.gov Identifier: NCT02111460.
Conflict of interest statement
Figures
Comment in
-
The Importance of Locoregional Therapy in Metastatic Nasopharyngeal Cancer.JAMA Oncol. 2020 Sep 1;6(9):1353-1354. doi: 10.1001/jamaoncol.2020.1793. JAMA Oncol. 2020. PMID: 32701124 No abstract available.
-
Chemoradiotherapy improves NPC outcomes.Nat Rev Clin Oncol. 2020 Oct;17(10):592. doi: 10.1038/s41571-020-0424-9. Nat Rev Clin Oncol. 2020. PMID: 32747764 No abstract available.
-
Locoregional Radiotherapy in Metastatic Nasopharyngeal Cancer-Reply.JAMA Oncol. 2021 Feb 1;7(2):311-312. doi: 10.1001/jamaoncol.2020.7017. JAMA Oncol. 2021. PMID: 33377962 No abstract available.
-
Locoregional Radiotherapy in Metastatic Nasopharyngeal Cancer.JAMA Oncol. 2021 Feb 1;7(2):310-311. doi: 10.1001/jamaoncol.2020.7011. JAMA Oncol. 2021. PMID: 33377965 No abstract available.
-
Locoregional Radiotherapy in Metastatic Nasopharyngeal Cancer.JAMA Oncol. 2021 Feb 1;7(2):310-311. doi: 10.1001/jamaoncol.2020.7014. JAMA Oncol. 2021. PMID: 33377966 No abstract available.
References
-
- GLOBOCAN cancer statistics. https://globocan.iarc.fr/Pages/fact_sheets population.aspx. Accessed January 2, 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous