Randomised clinical trial: tegoprazan, a novel potassium-competitive acid blocker, or lansoprazole in the treatment of gastric ulcer
- PMID: 32701188
- PMCID: PMC7496685
- DOI: 10.1111/apt.15865
Randomised clinical trial: tegoprazan, a novel potassium-competitive acid blocker, or lansoprazole in the treatment of gastric ulcer
Abstract
Background: Tegoprazan is a novel potassium-competitive acid blocker for the treatment of acid-related disorders.
Aims: To assess whether tegoprazan is non-inferior to lansoprazole in terms of efficacy and safety in patients with gastric ulcers.
Methods: In this phase 3, double-blind, active control, multicentre study, 306 gastric ulcer patients were randomised to one of three treatment groups: tegoprazan 50 mg, tegoprazan 100 mg and lansoprazole 30 mg once daily for 4 or 8 weeks. The primary endpoint was the cumulative proportion of patients with healed ulcers confirmed by endoscopy up to 8 weeks from treatment initiation. Symptoms and safety were assessed.
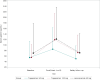
Results: In the full analysis set, the cumulative healing rates at week 8 were 94.8% (91/96) for the tegoprazan 50 mg, 95.0% (94/99) for the tegoprazan 100 mg and 95.7% (89/93) for the lansoprazole 30 mg groups. At week 4, the respective healing rates were 90.6% (87/96), 91.9% (91/99), and 89.2% (83/93). In per protocol analysis, 4-week healing rates were 95.4% (84/88), 94.6% (88/93) and 92.9% (79/85) for tegoprazan 50 mg, tegoprazan 100 mg and lansoprazole 30 mg, respectively. Both doses of tegoprazan were non-inferior to lansoprazole in ulcer healing at 4 and 8 weeks. The incidence of drug-related treatment-emergent adverse events did not differ among groups. The increase in serum gastrin concentration was not higher in tegoprazan-treated patients than in lansoprazole-treated patients.
Conclusions: Tegoprazan 50 or 100 mg were not inferior to lansoprazole 30 mg once daily in the treatment of gastric ulcers.
© 2020 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
Comment in
-
Editorial: tegoprazan-the newest advance in the management of acid-related diseases.Aliment Pharmacol Ther. 2020 Sep;52(6):1074-1075. doi: 10.1111/apt.15963. Aliment Pharmacol Ther. 2020. PMID: 33119154 No abstract available.
References
-
- Hunt RH, Cederberg C, Dent J, et al. Optimizing acid suppression for treatment of acid‐related diseases. Dig Dis Sci. 1995;40:24S‐49S. - PubMed
-
- Huang JQ, Hunt RH. Pharmacological and pharmacodynamic essentials of H2‐receptor antagonists and proton pump inhibitors for the practising physician. Best Pract Res Clin Gastroenterol. 2001;15:355‐370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical