Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma
- PMID: 32701411
- PMCID: PMC7655020
- DOI: 10.1200/JCO.20.01342
Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma
Abstract
Purpose: Chimeric antigen receptor (CAR) T-cell therapy of B-cell malignancies has proved to be effective. We show how the same approach of CAR T cells specific for CD30 (CD30.CAR-Ts) can be used to treat Hodgkin lymphoma (HL).
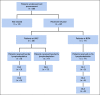
Methods: We conducted 2 parallel phase I/II studies (ClinicalTrials.gov identifiers: NCT02690545 and NCT02917083) at 2 independent centers involving patients with relapsed or refractory HL and administered CD30.CAR-Ts after lymphodepletion with either bendamustine alone, bendamustine and fludarabine, or cyclophosphamide and fludarabine. The primary end point was safety.
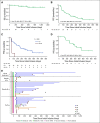
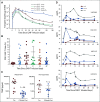
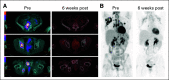
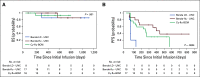
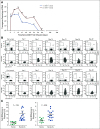
Results: Forty-one patients received CD30.CAR-Ts. Treated patients had a median of 7 prior lines of therapy (range, 2-23), including brentuximab vedotin, checkpoint inhibitors, and autologous or allogeneic stem cell transplantation. The most common toxicities were grade 3 or higher hematologic adverse events. Cytokine release syndrome was observed in 10 patients, all of which were grade 1. No neurologic toxicity was observed. The overall response rate in the 32 patients with active disease who received fludarabine-based lymphodepletion was 72%, including 19 patients (59%) with complete response. With a median follow-up of 533 days, the 1-year progression-free survival and overall survival for all evaluable patients were 36% (95% CI, 21% to 51%) and 94% (95% CI, 79% to 99%), respectively. CAR-T cell expansion in vivo was cell dose dependent.
Conclusion: Heavily pretreated patients with relapsed or refractory HL who received fludarabine-based lymphodepletion followed by CD30.CAR-Ts had a high rate of durable responses with an excellent safety profile, highlighting the feasibility of extending CAR-T cell therapies beyond canonical B-cell malignancies.
Figures


Comment in
-
CAR T cells are active in Hodgkin lymphoma.Nat Rev Clin Oncol. 2020 Oct;17(10):592. doi: 10.1038/s41571-020-0425-8. Nat Rev Clin Oncol. 2020. PMID: 32753735 No abstract available.
References
-
- Glimelius I, Ekberg S, Jerkeman M, et al. Long-term survival in young and middle-aged Hodgkin lymphoma patients in Sweden 1992-2009-trends in cure proportions by clinical characteristics. Am J Hematol. 2015;90:1128–1134. - PubMed
-
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet. 2002;359:2065–2071. - PubMed
-
- Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: A retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115:3671–3677. - PubMed
-
- Dürkop H, Latza U, Hummel M, et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell. 1992;68:421–427. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

