Postnatal maturation of spinal dynorphin circuits and their role in somatosensation
- PMID: 32701849
- PMCID: PMC7509208
- DOI: 10.1097/j.pain.0000000000001884
Postnatal maturation of spinal dynorphin circuits and their role in somatosensation
Abstract
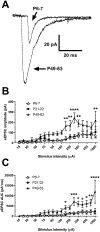
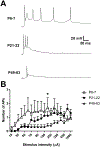
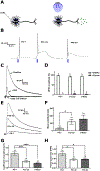
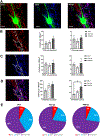
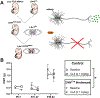
Inhibitory interneurons in the adult spinal dorsal horn (DH) can be neurochemically classified into subpopulations that regulate distinct somatosensory modalities. Although inhibitory networks in the rodent DH undergo dramatic remodeling over the first weeks of life, little is known about the maturation of identified classes of GABAergic interneurons, or whether their role in somatosensation shifts during development. We investigated age-dependent changes in the connectivity and function of prodynorphin (DYN)-lineage neurons in the mouse DH that suppress mechanosensation and itch during adulthood. In vitro patch clamp recordings revealed a developmental increase in primary afferent drive to DYN interneurons and a transition from exclusive C-fiber monosynaptic input to mixed A-fiber and C-fiber innervation. Although most adult DYN interneurons exhibited tonic firing as expected from their inhibitory phenotype, neonatal and adolescent DYN cells were predominantly classified as phasic or single-spiking. Importantly, we also found that most of the inhibitory presynaptic terminals contacting lamina I spinoparabrachial projection neurons (PNs) originate from DYN neurons. Furthermore, inhibitory synaptic input from DYN interneurons onto PNs was weaker during the neonatal period, likely reflecting a lower number of GABAergic terminals and a reduced probability of GABA release compared to adults. Finally, spinal DYN interneurons attenuated mechanical sensitivity throughout development, but this population dampened acute nonhistaminergic itch only during adulthood. Collectively, these findings suggest that the spinal "gates" controlling sensory transmission to the brain may emerge in a modality-selective manner during early life due to the postnatal tuning of inhibitory synaptic circuits within the DH.
Figures





References
-
- Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain 1994;56(1):95–101. - PubMed
-
- Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Developmental medicine and child neurology 1999;41(10):696–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

