The location of the axon initial segment affects the bandwidth of spike initiation dynamics
- PMID: 32701953
- PMCID: PMC7402515
- DOI: 10.1371/journal.pcbi.1008087
The location of the axon initial segment affects the bandwidth of spike initiation dynamics
Abstract
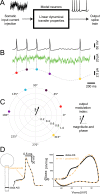
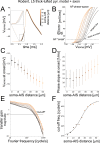
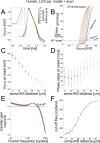
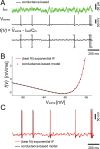
The dynamics and the sharp onset of action potential (AP) generation have recently been the subject of intense experimental and theoretical investigations. According to the resistive coupling theory, an electrotonic interplay between the site of AP initiation in the axon and the somato-dendritic load determines the AP waveform. This phenomenon not only alters the shape of APs recorded at the soma, but also determines the dynamics of excitability across a variety of time scales. Supporting this statement, here we generalize a previous numerical study and extend it to the quantification of the input-output gain of the neuronal dynamical response. We consider three classes of multicompartmental mathematical models, ranging from ball-and-stick simplified descriptions of neuronal excitability to 3D-reconstructed biophysical models of excitatory neurons of rodent and human cortical tissue. For each model, we demonstrate that increasing the distance between the axonal site of AP initiation and the soma markedly increases the bandwidth of neuronal response properties. We finally consider the Liquid State Machine paradigm, exploring the impact of altering the site of AP initiation at the level of a neuronal population, and demonstrate that an optimal distance exists to boost the computational performance of the network in a simple classification task.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

