Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy
- PMID: 32702095
- PMCID: PMC7454684
- DOI: 10.1093/infdis/jiaa446
Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy
Abstract
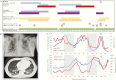
The antiviral drug remdesivir has been shown clinically effective for treatment of COVID-19. We here demonstrate suppressive but not curative effect of remdesivir in an immunocompromised patient. A man in his fifties treated with chemoimmunotherapy for chronic lymphocytic leukemia experienced a 9-week course of COVID-19 with high fever and severe viral pneumonia. During two 10-day courses of remdesivir starting 24 and 45 days after fever onset, pneumonia and spiking fevers remitted, but relapsed after discontinuation. Kinetics of temperature, C-reactive protein, and lymphocyte counts mirrored the remitting/relapsing SARS-CoV-2 infection. Combination therapy or longer treatment duration may be needed in immunocompromised patients.
Keywords: COVID-19; SARS-CoV-2; case report; immunocompromised; remdesivir.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

