Red cell membrane disorders: structure meets function
- PMID: 32702754
- PMCID: PMC7483429
- DOI: 10.1182/blood.2019000946
Red cell membrane disorders: structure meets function
Abstract
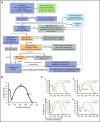
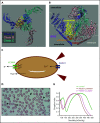
The mature red blood cell (RBC) lacks a nucleus and organelles characteristic of most cells, but it is elegantly structured to perform the essential function of delivering oxygen and removing carbon dioxide from all other cells while enduring the shear stress imposed by navigating small vessels and sinusoids. Over the past several decades, the efforts of biochemists, cell and molecular biologists, and hematologists have provided an appreciation of the complexity of RBC membrane structure, while studies of the RBC membrane disorders have offered valuable insights into structure-function relationships. Within the last decade, advances in genetic testing and its increased availability have made it possible to substantially build upon this foundational knowledge. Although disorders of the RBC membrane due to altered structural organization or altered transport function are heterogeneous, they often present with common clinical findings of hemolytic anemia. However, they may require substantially different management depending on the underlying pathophysiology. Accurate diagnosis is essential to avoid emergence of complications or inappropriate interventions. We propose an algorithm for laboratory evaluation of patients presenting with symptoms and signs of hemolytic anemia with a focus on RBC membrane disorders. Here, we review the genotypic and phenotypic variability of the RBC membrane disorders in order to raise the index of suspicion and highlight the need for correct and timely diagnosis.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Lux SE, Palek J. Disorders of the red cell membrane. In: Handin RI, Stossel TP, eds. Blood: Principles and Practice of Hematology Lippincott: Philadelphia, PA; 19951701-1818.
-
- Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23(1):787-818. - PubMed
-
- Sung LA, Vera C. Protofilament and hexagon: a three-dimensional mechanical model for the junctional complex in the erythrocyte membrane skeleton. Ann Biomed Eng. 2003;31(11):1314-1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

