Pathogenic Autoimmunity in Atherosclerosis Evolves From Initially Protective Apolipoprotein B100-Reactive CD4+ T-Regulatory Cells
- PMID: 32703007
- PMCID: PMC7515473
- DOI: 10.1161/CIRCULATIONAHA.119.042863
Pathogenic Autoimmunity in Atherosclerosis Evolves From Initially Protective Apolipoprotein B100-Reactive CD4+ T-Regulatory Cells
Abstract
Background: Throughout the inflammatory response that accompanies atherosclerosis, autoreactive CD4+ T-helper cells accumulate in the atherosclerotic plaque. Apolipoprotein B100 (apoB), the core protein of low-density lipoprotein, is an autoantigen that drives the generation of pathogenic T-helper type 1 (TH1) cells with proinflammatory cytokine secretion. Clinical data suggest the existence of apoB-specific CD4+ T cells with an atheroprotective, regulatory T cell (Treg) phenotype in healthy individuals. Yet, the function of apoB-reactive Tregs and their relationship with pathogenic TH1 cells remain unknown.
Methods: To interrogate the function of autoreactive CD4+ T cells in atherosclerosis, we used a novel tetramer of major histocompatibility complex II to track T cells reactive to the mouse self-peptide apo B978-993 (apoB+) at the single-cell level.
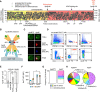
Results: We found that apoB+ T cells build an oligoclonal population in lymph nodes of healthy mice that exhibit a Treg-like transcriptome, although only 21% of all apoB+ T cells expressed the Treg transcription factor FoxP3 (Forkhead Box P3) protein as detected by flow cytometry. In single-cell RNA sequencing, apoB+ T cells formed several clusters with mixed TH signatures that suggested overlapping multilineage phenotypes with pro- and anti-inflammatory transcripts of TH1, T helper cell type 2 (TH2), and T helper cell type 17 (TH17), and of follicular-helper T cells. ApoB+ T cells were increased in mice and humans with atherosclerosis and progressively converted into pathogenic TH1/TH17-like cells with proinflammatory properties and only a residual Treg transcriptome. Plaque T cells that expanded during progression of atherosclerosis consistently showed a mixed TH1/TH17 phenotype in single-cell RNA sequencing. In addition, we observed a loss of FoxP3 in a fraction of apoB+ Tregs in lineage tracing of hyperlipidemic Apoe-/- mice. In adoptive transfer experiments, converting apoB+ Tregs failed to protect from atherosclerosis.
Conclusions: Our results demonstrate an unexpected mixed phenotype of apoB-reactive autoimmune T cells in atherosclerosis and suggest an initially protective autoimmune response against apoB with a progressive derangement in clinical disease. These findings identify apoB autoreactive Tregs as a novel cellular target in atherosclerosis.
Keywords: T-lymphocytes; T-lymphocytes, regulatory; apolipoprotein B-100; atherosclerosis; autoimmunity.
Figures






References
-
- Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

